thermosphere → termosfera
Thermosphere is the layer of the earth’s atmosphere extending from the top of the mesosphere (80 km - 90 km above the surface) to about 500 km. It is characterised by a rapid increase in temperature with increasing altitude up to about 200 km, followed by a levelling off in the 300 km - 500 km region.
thin layer chromatography → tankoslojna kromatografija
Thin layer chromatography. (TLC) is a technique for separating components in a mixture on the basis of their differing polarities. A spot of sample is placed on a flat sheet coated with silica and then carried along by a solvent that soaks the sheet. Different components will move different distances over the surface. TLC is a useful screening technique in clinical chemistry; for example, it can be used to detect the presence of drugs in urine.
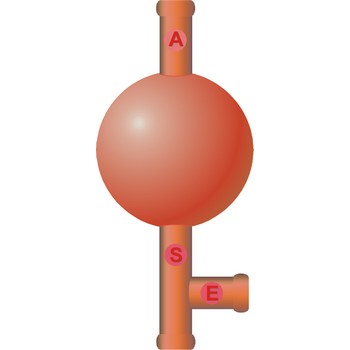
three way safety bulb → propipeta
Three way safety bulb (pipette filler bulb) is used for pipeting. The attachment is placed over the mouth of the pipet. Squeeze the air valve (A) and the bulb simultaneously to empty the bulb of air. Place the tip of the pipet below the solution's surface in the beaker. Gradually squeeze the suction valve (S) to draw liquid into the pipet. If the level of the solution is not high enough, squeeze the air valve (A) and the bulb again to expel the air from the bulb. Draw up more liquid by squeezing the suction valve (S). When the liquid is above the specified volume, stop squeezing the suction valve (S). Do not remove the bulb from the pipet. Do not allow liquid to enter the pipet bulb.
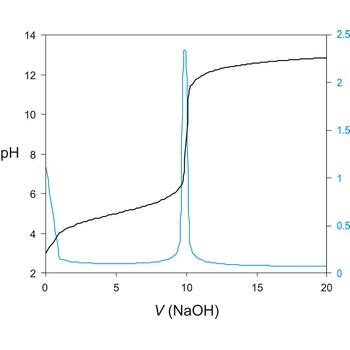
titration curve → titracijska krivulja
Titration curve is a graphic representation of the amount of a species present vs. volume of solution added during a titration. A titration curve has a characteristic sigmoid curve. The inflection point in the titration curve marks the end-point of the titration. Blue line is the first derivative of the titration curve.
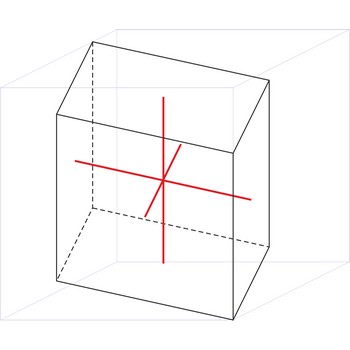
triclinic crystal system → triklinski kristalni sustav
Minerals of the triclinic crystal system are referred to three unequal axes, all of which intersect at oblique angles. None of the axes are perpendicular to any other axis.
a ≠ b ≠ c
α ≠ β ≠ γ ≠ 90°
triclinic lattice → triklinska rešetka
Triclinic lattice has one lattice point at the each corner of the unit cell. It has unit cell vectors a≠b≠c and interaxial angles α≠β≠γ≠90°.
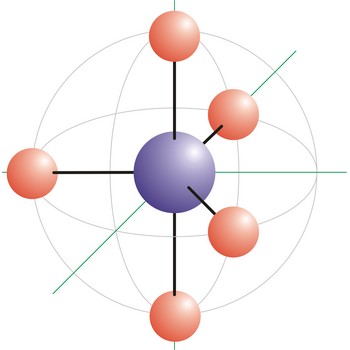
trigonal bipyramidal molecular geometry → trigonska bipiramidalna geometrija molekule
Trigonal bipyramidal (trigonal bipyramidal shape) is a molecular geometry that results when there are five bonds and no lone pairs on the central atom in the molecule. Three of the bonds are arranged along the atom’s equator, with 120° angles between them; the other two are placed at the atom’s axis. Axial bonds are at right angles to the equatorial bonds. Molecules with an trigonal bipyramidal electron pair geometries have sp3d (or dsp3) hybridization at the central atom. The PCl5 molecule has a trigonal bipyramidal molecular geometry.
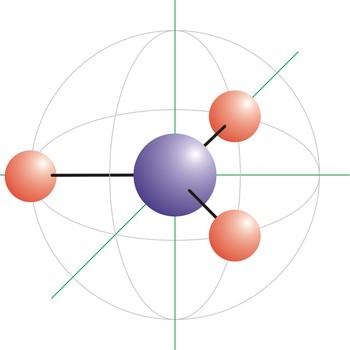
trigonal planar molecular geometry → trigonska planarna geometrija molekule
Trigonal planar is a molecular shape that results when there are three bonds and no lone pairs around the central atom in the molecule. The pairs are arranged along the central atom’s equator, with 120° angles between them. Molecules with an trigonal planar electron pair geometries have sp2d hybridization at the central atom. The carbonate ion (CO32-) has a trigonal planar geometry.
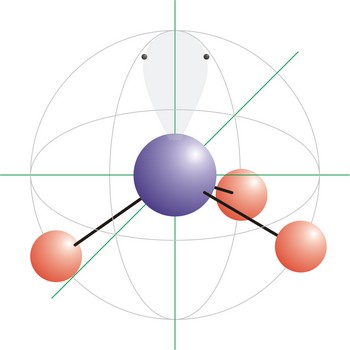
trigonal pyramidal molecular geometry → trigonska piramidalna geometrija molekule
Trigonal pyramidal is a molecular shape that results when there are three bonds and one lone pair on the central atom in the molecule. Molecules with an tetrahedral electron pair geometries have sp3 hybridization at the central atom. Ammonia (NH3) is a trigonal pyramidal molecule.
Citing this page:
Generalic, Eni. "Gallery/images.php." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table