glacial acetic acid → ledena octena kiselina
Glacial acetic acid (CH3COOH) is the pure compound, as distinguished from the usual water solutions known as acetic acid. It is a colorless liquid or crystalline substance (melting point 16.6 °C) with a pungent, vinegar odor.
Lewis acid → Lewisova kiselina
Lewis acid is an agent capable of accepting a pair of electrons to form a coordinate bond.
mineral acid → mineralna kiselina
Mineral acid is an acid made from minerals by chemical reaction, e.g. hydrochloric acid is produced from sodium chloride and sulphuric acid is made from sulphur.
monobasic acid → monobazična kiselina
Monobasic acids are acids that have only one replacable hydrogen atom per molecule (HCl, HNO3).
muriatic acid → murijatična kiselina
Muriatic acid is an obsolete name for hydrochloric acid (HCl). Lavoisier coined the name from the Latin word muria meaning brine.
organic acid → organska kiselina
Organic acid is an organic compound which is sour, most often these are carboxylic acids (RCOOH).
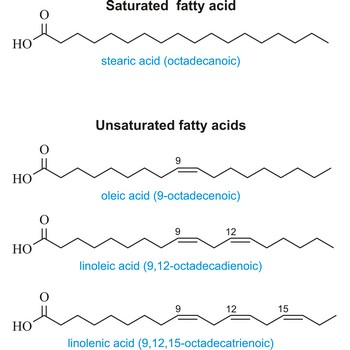
fatty acid → masna kiselina
Fatty acids are aliphatic monocarboxylic acids characterized by a terminal carboxyl group (R-COOH). The higher members of this series of acids occur in nature in the combined form of esters of glycerol (fats), and hence all acids of this family are called fatty acids. Natural fatty acids commonly have a chain of 4 to 28 carbons (usually unbranched and even-numbered), which may be saturated or unsaturated. The most important of saturated fatty acids are butyric (C4), lauric (C12), palmitic (C16), and stearic (C18). The most common unsaturated acids are oleic, linoleic, and linolenic (all C18).
The physical properties of fatty acids are determined by the chain length, degree of unsaturation, and chain branching. Short-chain acids are pungent liquids, soluble in water. As the chain length increases, melting points are raised and water-solubility decreases. Unsaturation and chain branching tend to lower melting points.
weak acid → slaba kiselina
Weak acid is an acid that incompletely dissociated in aqueous solution. Acetic acid is an example of a weak acid
glutamic acid → glutaminska kiselina
Glutamic acid is an electrically charged amino acids. It is one of the two amino acids that contain a carboxylic acid group in its side chains. These acids play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins. Glutamic acid is commonly referred to as glutamate, because its carboxylic acid side chain will be deprotonated and thus negatively charged in its anionic form at physiological pH. Glutamic acid is referred to as a non-essential amino acid because a healthy human can synthesize all the glutamic acid needed for normal body function from other amino acids.
- Abbreviations: Glu, E
- IUPAC name: 2-aminopentanedioic acid
- Molecular formula: C5H9NO4
- Molecular weight: 147.13 g/mol
lactic acid → mliječna kiselina
Lactic acid is an acid produced as a result of anaerobic respiration in muscles and red blood cells, i.e. when glycogen is used as an energy source for respiration rather than oxygen. After production, it is converted back to glycogen in the liver. The build up of large amounts of lactic acid in the blood can lead to stress and toxic effects. High levels are usually a result of sustained, excessive exercise.
Citing this page:
Generalic, Eni. "Dezoksiribonukleinska kiselina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table