mutarotation → mutarotacija
Mutarotation is the change in optical rotation accompanying epimerization. In carbohydrate chemistry this term usually refers to epimerization at the hemiacetal carbon atom. In general α- and β-form are stable solids, but in solution they rapidly equilibrate. For example, D-glucose exists in an equilibrium mixture of 36 % α-D-glucopyranose and 64 % β-D-glucopyranose, with only a tiny fraction in the open-chain form. The equilibration occurs via the ring opening of the cyclic sugar at the anomeric center with the acyclic form as the intermediate. Mutarotation was discovered by French chemist Augustin-Pierre Dubrunfaut (1797-1881) in 1846.
monosaccharide → monosaharid
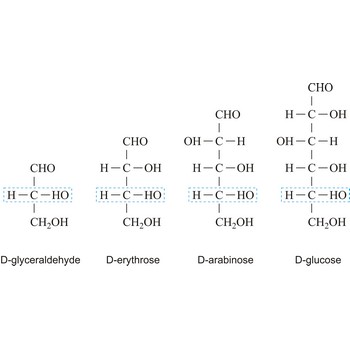
Monosaccharides are carbohydrates, with the general formula Cn(H2O)n, that cannot be decomposed to a simpler carbohydrates by hydrolysis.
Depending on whether the molecule contains an aldehyde group (-CHO) or a ketone group (-CO-) monosaccharide can be a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose). These aldehyde and ketone groups confer reduction properties on monosaccharides. They are also classified according to the number of carbon atoms they contain: trioses have three carbon atoms, tetroses four, pentoses five, hexoses six, heptoses seven, etc. These two systems of classification are often combined. For example, a six-carbon polyhydroxy aldehyde such as D-glucose is an aldohexose, whereas a six-carbon polyhydroxy ketone such as D-fructose is a ketohexose.
The notations D and L are used to describe the configurations of carbohydrates. In Fischer projections of monosaccharides, the carbonyl group is always placed on top (in the case of aldoses) or as close to the top as possible (in the case of ketoses). If the OH group attached to the bottom-most asymmetric carbon (the carbon that is second from the bottom) is on the right, then the compound is a D-sugar. If the OH group is on the left, then the compound is an L-sugar. Almost all sugars found in nature are D-sugars.
Monosaccharides can exist as either straight-chain or ring-shaped molecules. During the conversion from straight-chain form to cyclic form, the carbon atom containing the carbonyl oxygen, called the anomeric carbon, becomes a chiral center with two possible configurations (anomers), α and β. When the stereochemistry of the first carbon matches the stereochemistry of the last stereogenic center the sugar is the α-anomer when they are opposite the sugar is the β-anomer.
proline → prolin
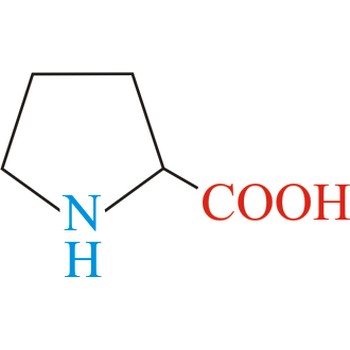
Proline has an aliphatic side chain with a distinctive cyclic structure. It is unusual because it is conformationally restricted. The secondary amino (imino) group of proline residues is held in a rigid conformation that reduces the structural flexibility of polypeptide regions containing proline. It is not an essential amino acid, which means that the human body can synthesize it.
- Abbreviations: Pro, P
- IUPAC name: pyrrolidine-2-carboxylic acid
- Molecular formula: C5H9NO2
- Molecular weight: 115.13 g/mol
ringlike structure → prstenasta struktura
The ringlike structure is a structure by which the cycloalcanes and aromates are shown.
Citing this page:
Generalic, Eni. "Cyclic." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table