ionisation → ionizacija
Ionisation is the process of producing ions. Certain molecules ionise in a solution; for example, acids ionise when dissolved in water.
Electron transfer also causes ionisation in certain reactions, for example sodium and chlorine react by transfer of a valence electron from the sodium atom to the chlorine atom to form the ions that constitute a sodium chloride crystal.
lysine → lizin
Lysine is an electrically charged amino acids with basic side chains. Lysine is a base, as are arginine and histidine. The amino group is highly reactive and often participates in reactions at the active centers of enzymes. Lysine plays an important role in coordinating negatively charged ligands. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Lys, K
- IUPAC name: 2,6-diaminohexanoic acid
- Molecular formula: C6H14N2O2
- Molecular weight: 146.19 g/mol
metallic glass → metalno staklo
Certain alloys can solidify by extremely rapid cooling out of melt without formation of a crystal lattice, that is in the amorphous form - such, amorphous alloys are so called metallic glasses. The alloy of zirconium, beryllium, titanium, copper, and nickel is one of the first metallic glasses that can be made in bulk and formed into strong, hard, useful objects.
Unlike pure metals and most metal alloys, metallic glasses have no regular crystalline structure. This lack of long range order or microstructure is related to such desirable features as strength and low damping which is one reason why the premier use for zirconium-based metallic glass is in the manufacture of expensive golf club heads. Metallic glasses can be quite strong yet highly elastic, and they can also be quite tough (resistant to fracture). Even more interesting are the thermal properties; for instance, just like an oxide glass, there is a temperature (called the glass transition temperature) above which a metallic glass becomes quite soft and flows easily. This means that there are lots of opportunities for easily forming metallic glasses into complex shapes.
methyl orange → metil oranž
Methyl orange is an acid-base indicator, in acid it turns red and in a base it turns yellow.
polymorphism → polimorfija
Polymorphism is the ability of a solid substance to crystallise into more than one different crystal structure. Different polymorphs have different arrangements of atoms within the unit cell, and this can have a profound effect on the properties of the final crystallised compound. The change that takes place between crystal structures of the same chemical compound is called polymorphic transformation.
The set of unique crystal structures a given compound may form are called polymorphs. Calcium carbonate is dimorphous (two forms), crystallizing as calcite or aragonite. Titanium dioxide is trimorphous; its three forms are brookite, anatase, and rutile. The prevailing crystal structure depends on both the temperature and the external pressure.
Iron is a metal with polymorphism structure. Each structure stable in the range of temperature, for example, when iron crystallizes at 1 538 °C it is bcc (δ-iron), at 1 394 °C the structure changes to fcc (γ-iron or austenite), and at 912 °C it again becomes bcc (α-iron or ferrite).
Polymorphism of an element is called allotropy.
potentiometric titration → potenciometrijska titracija
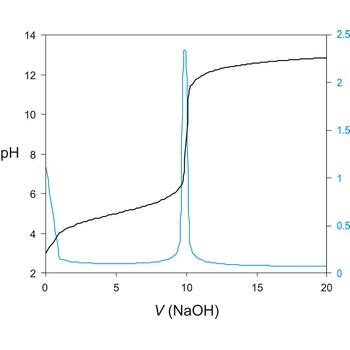
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
X-ray diffraction → rendgenska difrakcija
The regular array of atoms in a crystal is a three-dimensional diffraction grating for short-wavelength waves such as X-rays. The atoms are arranged in planes with interplanar spacing d. Diffraction maxima occur in the incident direction of the wave, measured from the surface of a plane of atoms, and the wavelength λ of the radiation satisfy Braggs’s law:
zwitterion → dipolarni ion
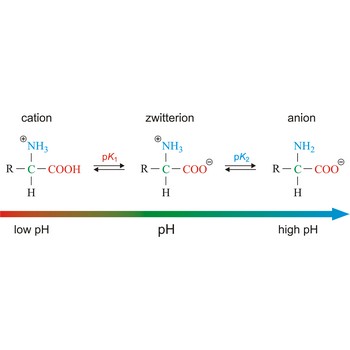
Zwitterion, also known as inner salt or dipolar ion, is an ion with a positive and a negative electrical charge at different locations within a molecule. As the molecule contains two opposite charges, it is electrically neutral. The term zwitterion is derived from the German word zwitter, meaning a hybrid, hermaphrodite. Zwitterions can be formed from compounds that contain both acid groups and base groups in their molecules (ampholytes).
All of the common amino acids found in proteins are ampholytes because they contain a carboxyl group (-COOH) that acts as an acid and an amino group (-NH2) that acts as a base. In the solid state, amino acids exist in the dipolar or zwitterion form. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (H+) ion, and the amino acid becomes positively charged. If base is added, ion removal of the H+ ion from the amino group of the zwitterion produces a negatively charged amino acid.
Citing this page:
Generalic, Eni. "Bazno centrirana ortorompska rešetka." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table