unsaturated fat → nezasićena mast
Unsaturated fats, which include one or more unsaturated fatty acid, are liquid at room temperature (oil) and come from plant oils such as olive, peanut, corn, sunflower, safflower, and soybean. The fish oils may also be high in unsaturated fatty acids.
uranium → uranij
Uranium was discovered by Martin Heinrich Klaproth (Germany) in 1789. Named after the planet Uranus. It is silvery-white, dense, ductile, malleable, radioactive metal. Resists alkalis; tarnishes in air; attacked by steam and acids. Radiotoxic. Uranium occurs in many rocks, but in large amounts only in such minerals as pitchblende and carnotite. For many centuries it was used as a pigment for glass. Now it is used as a fuel in nuclear reactors and in bombs.
valine → valin
Valine is hydrophobic amino acids with aliphatic side chain. It is a member of the branched-chain amino acid family, along with leucine and isoleucine. Valine differs from threonine by replacement of the hydroxyl group with a methyl substituent, but they are of roughly the same shape and volume. The nonpolar hydrophobic amino acids tend to cluster together within proteins, stabilizing protein structure by means of hydrophobic interactions. Valine is an essential amino acid, which means that it cannot be synthesized in the body and must be obtained through dietary sources.
- Abbreviations: Val, V
- IUPAC name: 2-amino-3-methylbutanoic acid
- Molecular formula: C5H11NO2
- Molecular weight: 117.15 g/mol
vanadium → vanadij
Vanadium was discovered by A. M. del Rio (Spain) in 1801 and rediscovered by Nils Sefstrom (Sweden) in 1830. Named after Vanadis, the goddess of beauty in Scandinavian mythology. It is soft, ductile, silvery-white metal. Resistant to corrosion by moisture, air and most acids and alkalis at room temperature. Exposed surfaces form oxide coating. Reacts with concentrated acids. Vanadium is found in the minerals patronite (VS4), vanadinite [Pb5(VO4)3Cl] and carnotite [K2(UO2)2(VO4)2·3H2O]. Pure metal produced by heating with C and Cl to produce VCl3 which is heated with Mg in Ar atmosphere. It is mixed with other metals to make very strong and durable alloys. Vanadium pentoxide (V2O5) is used as a catalyst, dye and fixer-fixer.
volumetric flask → odmjerna tikvica
Volumetric flasks are bottles made of glass, in a pear like in shape with long thin necks and flat bottoms. All come with a ground glass stopper for a tight seal. Volume marking is cut in glass with fluoride acid around the neck, so that parallax should be avoided (flask is put in front of the eyes so that one can see only a straight horizontal line). A volumetric flask is calibrated to contain (TC or In) the indicated volume of water at 20 °C when the bottom of the meniscus is adjusted to just rest on the center of the line marked on the neck of the flask. They are used for preparing the exactly known volume of sample solution and standard solutions of reagents. On each flask with volume designation a temperature on which the flask has been calibrated is designated.
ytterbium → iterbij
Ytterbium was discovered by Jean de Marignac (France) in 1878. Named after Ytterby, a village in Sweden. It is silvery, lustrous, malleable and ductile metal. Oxidizes slowly in air. Reacts with water. Flammable dust. Ytterbium is found in minerals such as yttria, monazite, gadolinite and xenotime. Used in metallurgical and chemical experiments.
zinc → cink
Zinc was discovered by Andreas Marggraf (Germany) in 1746. The origin of the name comes from the German word zink. It is bluish-silver, ductile metal. Reacts with alkalis and acids. Tarnishes in air. Zinc is found in the minerals zinc blende (sphalerite) (ZnS), calamine, franklinite, smithsonite (ZnCO3), willemite and zincite (ZnO). Used to coat other metal (galvanizing) to protect them from rusting. Although some 90 % of the zinc is used for galvanizing steel. Zinc metal is used in the common dry-cell battery. Also used in alloys such as brass, bronze. Zinc compounds are also used in the manufacture of paints, cosmetics, plastics, electronic devices, and other products.
zirconium → cirkonij
Zirconium was discovered by Martin Heinrich Klaproth (Germany) in 1789. The origin of the name comes from the Arabic word zargun meaning gold colour. It is grey-white, lustrous, corrosion-resistant metal. Exposed surfaces form oxide protective film. Zirconium is found in many minerals such as zircon and baddeleyite. Used in alloys such as zircaloy this is used in nuclear applications since it does not readily absorb neutrons. Also baddeleyite is used in lab crucibles. Used in high-performance pumps and valves. Clear zircon (ZrSiO4) is a popular gemstone.
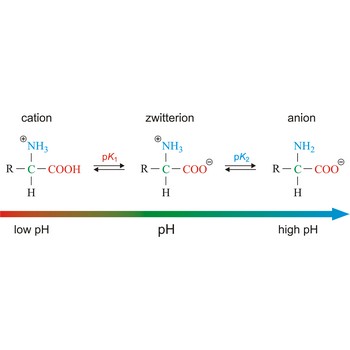
zwitterion → dipolarni ion
Zwitterion, also known as inner salt or dipolar ion, is an ion with a positive and a negative electrical charge at different locations within a molecule. As the molecule contains two opposite charges, it is electrically neutral. The term zwitterion is derived from the German word zwitter, meaning a hybrid, hermaphrodite. Zwitterions can be formed from compounds that contain both acid groups and base groups in their molecules (ampholytes).
All of the common amino acids found in proteins are ampholytes because they contain a carboxyl group (-COOH) that acts as an acid and an amino group (-NH2) that acts as a base. In the solid state, amino acids exist in the dipolar or zwitterion form. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (H+) ion, and the amino acid becomes positively charged. If base is added, ion removal of the H+ ion from the amino group of the zwitterion produces a negatively charged amino acid.
Schrotter apparatus for determination of CO2 → Schrotterova aparatura za određivanje CO2
Schrötter decomposition apparatus (Schrötter's alkalimeter) is used to determining the carbonate content in samples of limestone, gypsum, dolomite, or baking powder by loss of weight. The apparatus is named after the Austrian chemist Anton Schrötter von Kristelli (1802-1875), who devised it in 1871. The size of the filled apparatus (apparatus is 16 cm high) is such that it weights less than 75 g, and can be placed on the pan of an analytical balance.
Procedure: Weigh about 0.5 g of the powdered carbonate sample and introduce it into the decomposition flask C. Pour into the drying tube A 2-3 mL of concentrated sulphuric acid (H2SO4), and to the dropping funnel B add about 10-15 mL of hydrochloric acid (w(HCl) = 15 %). Weigh the whole apparatus. Open the upper taps of both parts and allow the hydrochloric acid from B to run slowly down on to the powdered sample. The evolved CO2 escapes through the strong sulphuric acid and is thus thoroughly dried. When further addition of acid produces no more evolution of CO2, warm the apparatus up to 80 °C so as to expel the CO2 from the solution. Connect the upper tap of the drying tube A to a water pump and draw a slow current of air through the apparatus until completely cool. Open the upper taps for a moment to equalize the internal and external pressure and weight the apparatus again. The weight loss is equal to the weight of carbon dioxide liberated from the carbonates.
Citing this page:
Generalic, Eni. "Asparaginska kiselina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table