unit cell → jedinična ćelija
Unit cell is the smallest fragment of the structure of a solid that by repetition can generate the entire structure.
monosaccharide → monosaharid
Monosaccharides are carbohydrates, with the general formula Cn(H2O)n, that cannot be decomposed to a simpler carbohydrates by hydrolysis.
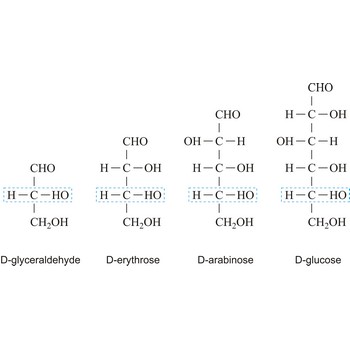
Depending on whether the molecule contains an aldehyde group (-CHO) or a ketone group (-CO-) monosaccharide can be a polyhydroxy aldehyde (aldose) or a polyhydroxy ketone (ketose). These aldehyde and ketone groups confer reduction properties on monosaccharides. They are also classified according to the number of carbon atoms they contain: trioses have three carbon atoms, tetroses four, pentoses five, hexoses six, heptoses seven, etc. These two systems of classification are often combined. For example, a six-carbon polyhydroxy aldehyde such as D-glucose is an aldohexose, whereas a six-carbon polyhydroxy ketone such as D-fructose is a ketohexose.
The notations D and L are used to describe the configurations of carbohydrates. In Fischer projections of monosaccharides, the carbonyl group is always placed on top (in the case of aldoses) or as close to the top as possible (in the case of ketoses). If the OH group attached to the bottom-most asymmetric carbon (the carbon that is second from the bottom) is on the right, then the compound is a D-sugar. If the OH group is on the left, then the compound is an L-sugar. Almost all sugars found in nature are D-sugars.
Monosaccharides can exist as either straight-chain or ring-shaped molecules. During the conversion from straight-chain form to cyclic form, the carbon atom containing the carbonyl oxygen, called the anomeric carbon, becomes a chiral center with two possible configurations (anomers), α and β. When the stereochemistry of the first carbon matches the stereochemistry of the last stereogenic center the sugar is the α-anomer when they are opposite the sugar is the β-anomer.
nuclear magnetic resonance → nuklearna magnetska rezonancija
Nuclear magnetic resonance (NMR) is a type of radio-frequency spectroscopy based on the magnetic field generated by the spinning of electrically charged atomic nuclei. This nuclear magnetic field is caused to interact with a very large (1 T - 5 T) magnetic field of the instrument magnet. NMR techniques have been applied to studies of electron densities and chemical bonding and have become a fundamental research tool for structure determinations in organic chemistry.
vitamin → vitamin
The name vitamin is obtained from vital amines as it was originally thought that these substances were all amines. This is now known not to be true as vitamins have a range of structures. The body requires a small amout of vitamins, but any deficiency leads to metabolic and physical disorders.
X-ray diffraction pattern → rendgenski difrakcijski uzorci
X-ray diffraction pattern is an interference pattern created by x-rays as they pass through a solid material. Studying X-ray diffraction patterns gives detailed information on the three-dimensional structure of crystals, surfaces, and atoms.
nucleotide → nukleotid
Nucleotides are the components that made up nucleic acids. They have three major components: the first component is a nitrogenous base, which is derivative of one of two parent compounds, pyrimidine or purine; the second is a pentose, or five carbon sugar group; the third is a unit of phosphate. Each group of three nucleotides in a gene is known as a codon. Whenever the phosphate group is not present, a nucleotide becomes a nucleoside.
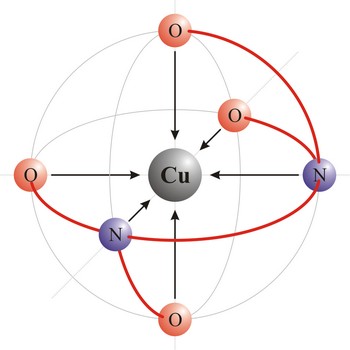
polydentant ligand → polidentantni liganad
Polydentant ligands contain more co-ordination points (can give more electron pairs) and they form complex ringlike structures (celate complexes) by replacing two or more monodentant ligands. That kind of ligand is EDTA which has 6 co-ordinational points and with metals it creates complexes, always in 1:1 ratio.
polymer → polimer
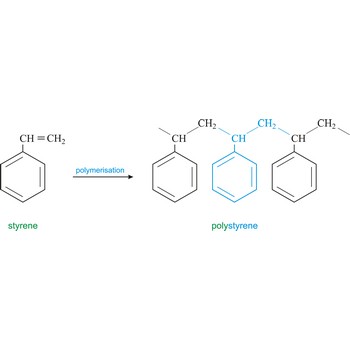
Polymer is a substance composed of molecules of high relative molecular mass (molecular weight), the structure of which essentially comprises the multiple repetition of units derived, actually or conceptually, from molecules of low relative molecular mass (monomers). In most cases the number of monomers is quite large and is often not precisely known. A single molecule of a polymer is called a macromolecule. Polystyrene is light solid material obtained by polymerisation of styrene (vinyl benzene).
Citing this page:
Generalic, Eni. "Lewisova struktura." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table