supercritical carbon dioxide → superkritični ugljikov dioksid
Supercritical carbon dioxide (scCO2) is a powerful, cheap, non-toxic and environmental friendly solvent. When used at a supercritical state (over 74 bar and 31 °C), it achieves similar solvating power as its organic competitors, such as hydrocarbons and chlorinated solvents. Supercritical carbon dioxide is one of few solvents that can be unrestrictedly used for food processing.
starch → škrob
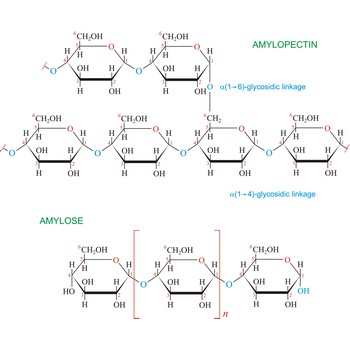
Starch (C6H10O5)x is a polysaccharide used by plants to stockpile glucose molecules. It is the major component of flour, potatoes, rice, beans, corn, and peas. Starch is a mixture of two different polysaccharides: amylose (about 20 %), which is insoluble in cold water, and amylopectin (about 80 %), which is soluble in cold water. Amylose is composed of unbranched chains of D-glucose units joined by α(1→4)-glycosidic linkages. Unlike amylose, which are linear polymers, amylopectin contains α(1→6)-glycoside branches approximately every 25 glucose units.
Starch digestion begins in the mouth via the action of amylase, a digestive enzyme present in saliva. The process is completed in the small intestine by the pancreatic amylase. The final products of starch digestion, glucose molecules, are absorbed into the intestinal bloodstream and transported to the liver. Like most enzymes, glycosidases are highly selective in their action. They hydrolyze only the α-glycoside links in starch and leave the β-glycoside links in cellulose untouched. Starch is important food stuff and is used in adhesives, and sizes, in laundering, pharmacy and medicine.
thermite → termit
Thermite is a stoichiometric powdered mixture of iron(II) oxide and aluminium for the reaction
The reaction is highly exothermic and the increase in temperature (over 2500 °C) is sufficient to melt the iron produced. It has been used for localized welding of steel object (e.g. railway lines) in the thermit process. Thermite is also used in incendiary bombs.
thermodynamic laws → termodinamički zakoni
Thermodynamic laws are the foundation of the science of thermodynamics:
First law: The internal energy of an isolated system is constant; if energy is supplied to the system in the form of heat dq and work dw, then the change in energy dU = dq + dw.
Second law: No process is possible in which the only result is the transfer of heat from a reservoir and its complete conversion to work.
Third law: The entropy of a perfect crystal approaches zero as the thermodynamic temperature approaches zero.
tryptophan → triptofan
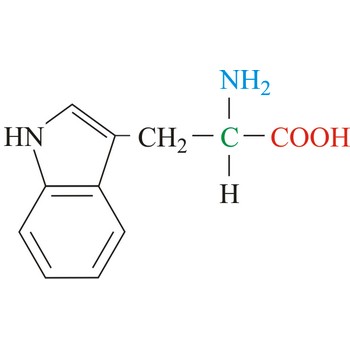
Tryptophan is hydrophobic amino acids with aromatic side chain. Tryptophan is large aromatic residue that is normally found buried in the interior of a protein and is important for protein stability. Tryptophan has the largest side chain and is the least common amino acid in proteins. It has spectral properties that make it the best inherent probe for following protein folding and conformational changes associated with biochemical processes. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Trp, W
- IUPAC name: 2-amino-3-(1H-indol-3-yl)propanoic acid
- Molecular formula: C11H12N2O2
- Molecular weight: 204.23 g/mol
Schrotter apparatus for determination of CO2 → Schrotterova aparatura za određivanje CO2
Schrötter decomposition apparatus (Schrötter's alkalimeter) is used to determining the carbonate content in samples of limestone, gypsum, dolomite, or baking powder by loss of weight. The apparatus is named after the Austrian chemist Anton Schrötter von Kristelli (1802-1875), who devised it in 1871. The size of the filled apparatus (apparatus is 16 cm high) is such that it weights less than 75 g, and can be placed on the pan of an analytical balance.
Procedure: Weigh about 0.5 g of the powdered carbonate sample and introduce it into the decomposition flask C. Pour into the drying tube A 2-3 mL of concentrated sulphuric acid (H2SO4), and to the dropping funnel B add about 10-15 mL of hydrochloric acid (w(HCl) = 15 %). Weigh the whole apparatus. Open the upper taps of both parts and allow the hydrochloric acid from B to run slowly down on to the powdered sample. The evolved CO2 escapes through the strong sulphuric acid and is thus thoroughly dried. When further addition of acid produces no more evolution of CO2, warm the apparatus up to 80 °C so as to expel the CO2 from the solution. Connect the upper tap of the drying tube A to a water pump and draw a slow current of air through the apparatus until completely cool. Open the upper taps for a moment to equalize the internal and external pressure and weight the apparatus again. The weight loss is equal to the weight of carbon dioxide liberated from the carbonates.
Citing this page:
Generalic, Eni. "Carnotov kružni proces." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table