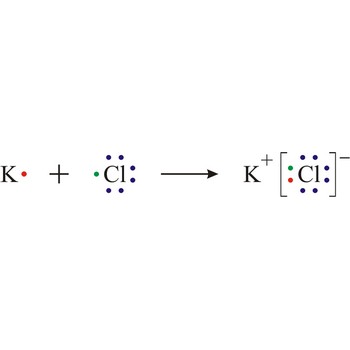glucose → glukoza
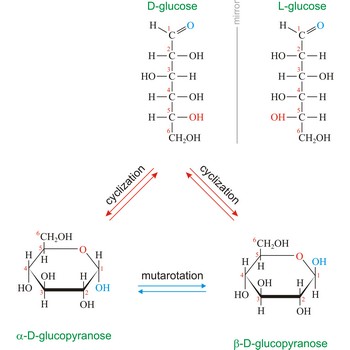
Glucose (grape sugar, blood sugar), C6H12O6, is an aldohexose (a monosaccharide sugar having six carbon atoms and an aldehyde group). An older common name for glucose is dextrose, after its dextrorotatory property of rotating plane polarized light to the right. Glucose in free (in sweet fruits and honey) or combined form (sucrose, starch, cellulose, glycogen) is is probably the most abundant organic compound in nature. During the photosynthesis process, plants use energy from the sun, water from the soil and carbon dioxide gas from the air to make glucose. In cellular respiration, glucose is ultimately broken down to yield carbon dioxide and water, and the energy from this process is stored as ATP molecules (36 molecules of ATP across all processes).
Naturally occurring glucose is D isomers (OH group on the stereogenic carbon farthest from the aldehyde group, C-5, is to the right in the Fischer projection). Although often displayed as an open chain structure, glucose and most common sugars exist as ring structures. In the α form, the hydroxyl group attached to C-1 and the CH2OH attached to C-5 are located on opposite sides of the ring. β-glucose has these two groups on the same side of the ring. The full names for these two anomers of glucose are α-D-glucopyranose and β-D-glucopyranose.
gravimetry → gravimetrija
Gravimetry is the quantitative measurement of an analyte by weighing a pure, solid form of the analyte. Since gravimetric analysis is an absolute measurement, it is the principal method for analysing and preparing primary standards.
A typical experimental procedure to determine an unknown concentration of an analyte in a solution is as follows:
- quantitatively precipitate the analyte from the solution
- collect the precipitate by filtering and wash it to remove impurities
- dry the solid in an oven to remove the solvent
- weigh the solid on an analytical balance
- calculate the analyte concentration in the original solution based on the weight of the precipitate.
regeneration → regeneracija
Regeneration is the process of restoring an ion exchange medium to a usable state after exhaustion. The cation exchanger is normally regenerated with hydrochloric acid and the anion exchanger with sodium hydroxide.
resolution → rezolucija
Resolution is a process by which a racemic mixture is separated into its two pure enantiomers.
reverse osmosis → reverzna osmoza
Reverse osmosis is the method used for obtaining freshwater from saltwater. The process uses a semi-permeable membrane through which pure water and not the salts will pass. The saltwater must be pressurised to approximately 25 bar, which makes it operationally expensive to produce large quantities of fresh water by this method.
saponification → saponifikacija
Saponification is a proces of hydrolysis of esters using hot sodium hydroxide solution to produce the salt of a carboxylic acid. Saponification usually refers to the hydrolysis of esters of fatty acids to manufacture soaps.
half-life → vrijeme poluraspada
For a simple radioactive decay process, half-life, t1/2, is defined as the time required for the activity of a given radioactive isotopes to decrease to half its value by that process.
The half-life is a characteristic property of each radioactive isotope and is independent of its amount or condition.
hydration → hidratacija
Hydration is addition of water or the elements of water (i.e. H and OH) to a molecular entity. The term is also used in a more restricted sense for the process:
ionic bond → ionska veza
Ionic bond is a strong force of attraction holding atoms together in a molecule or crystal. Typically chemical bonds have energies of about 100 kJ mol-1. Ionic bond is a bond at which one of the participants, during the procedure of bonding, gives away its unpaired electrons to another atom so that both can achieve electron arrangement of the closest noble gas. In order to form an ionic bond one of the atoms must cross to the positively charged ion by losing certain number of electrons and the other atom must receive those electrons and cross to the negatively charged ion.
Citing this page:
Generalic, Eni. "Carnotov kružni proces." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table