Dalton’s law → Daltonov zakon
Dalton’s law of partial pressure says that the total pressure eof gaseous mixture is equal to the sum of all gases partial pressures which make that mixture on the condition that they do not interact.
For example, if dry oxygen gas at 900 hPa is saturated with water vapor at 56 hPa, the pressure of the wet gas is 956 hPa.
law of chemical equilibrium → zakon o kemijskoj ravnoteži
Law of chemical equilibrium (also called the law of mass action) states that the rate at which a substance reacts is proportional to its active mass (i.e. to its molar concentration). Thus, the velocity of a chemical reaction is proportional to the product of the concentration of the reactants.
law of conservation of energy → zakon o očuvanju energije
Law of conservation of energy: In an isolated system energy can be transferred from one form to another but the total energy of the system remains constant.
Gauss’ law for electrostatics → Gaussov zakon za elektrostatiku
Gauss’ law describes the relation between charge and electric field in static situations, so it is equivalent to Coulomb’s law, which can be derived from Gauss’ law. Gauss’ law states that the net flux of electric field, Φ, through an imaginary closed surface, S, - a Gaussian surface - is equal to the net charge, q, inside that closed surface:
where electric flux Φ through Gaussian surface is given by:
ε0 is the permittivity constant and dS is a surface element.
Gibbs phase rule → Gibbsov zakon faza
Gibbs phase rule is the relationship used to determine the number of state variables, usually chosen from among temperature, pressure, and species composition in each phase, which must be specified to fix the thermodynamic state of a system in equilibrium:
where C is the number of components in a mixture, P is the number of phases, and F is the degrees of freedom, i.e., the number of intensive variables that can be changed independently without affecting the number of phases.
Graham’s law → Grahamov zakon
Graham’s law is the rates at whish gases diffuse are inversely proportional to the square roots of their densities. This principle is made use of in the diffusion method of separating isotopes. The law was formulated in 1829 by British chemist Thomas Graham (1805-1869).
Raoult’s law → Raoultov zakon
Raoult’s law is the expression for the vapour pressure pA of component A in an ideal solution, viz.,
where xA is the mole fraction of component A and pAo the vapour pressure of the pure substance A.
zero law of thermodynamics → nulti zakon termodinamike
Zero law of thermodynamics states: If some body A is in thermal equilibrium with body B and with body C, then bodies B and C are also in thermal equilibrium.
Hesse’s law → Hessov zakon
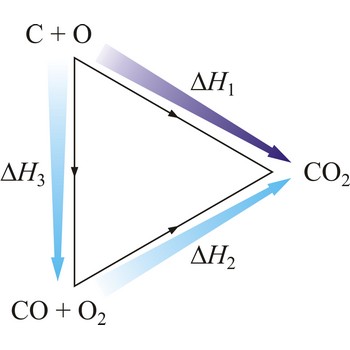
Hesse’s law says that reaction heat of some chemical change does not depend on the way in which the reaction is conducted, but only on starting and ending system state. Hesse’s law is also known as the law of constant heat summation. Hesse’s law is also known as the law of constant heat summation. The law was first put forward in 1840 by the Swiss-born Russian chemist Germain Henri Hess (1802-1850).
Hesse’s law can be used to obtain thermodynamic data that cannot be measured directly. For example, it is very difficult to control the oxidation of graphite to give pure CO. However, enthalpy for the oxidation of graphite to CO2 can easily be measured. So can the enthalpy of oxidation of CO to CO2. The application of Hess’s law enables us to estimate the enthalpy of formation of CO.
| C(s) + O2(g) →← CO2(g) | ΔrH1 = -393 kJ mol-1 |
| CO(g) + 1/2O2(g) →← CO2(g) | ΔrH2 = -283 kJ mol-1 |
| C(s) + 1/2O2(g) →← CO(g) | ΔrH3 = -110 kJ mol-1 |
The equation shows the standard enthalpy of formation of CO to be -110 kJ/mol.
Citing this page:
Generalic, Eni. "Zakon o određenom sastavu." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table