enthalpy growth → prirast entalpije
Enthalpy growth (ΔH) is that part of energy of system which can be translated into heat (Q) with a permanent pressure.
change of state → promjena stanja
Change of state is a physical change which appears when a substance crosses from one state into another. This usually happens because of the change of energy of particles provoked by heating or cooling.
chemical potential → kemijski potencijal
For a mixture of substances, the chemical potential of constituent B (μB) is defined as the partial derivative of the Gibbs energy G with respect to the amount (number of moles) of B, with temperature, pressure, and amounts of all other constituents held constant.
Also called partial molar Gibbs energy. Components are in equilibrium if their chemical potentials are equal.
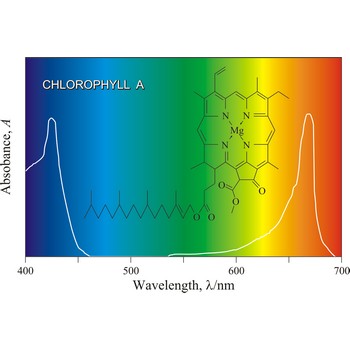
chlorophyll → klorofil
Chlorophyll is a green pigment present in green plants and cyanobacteria. Chlorophyll is essential in the transformation of light energy to chemical energy in photosynthesis. Chlorophyll absorbs light mostly in the blue and red ends of the visible spectrum, and very little in the green wavelengths. That green light is reflected, giving us the leaf colour we see.
Fermi level → Fermijev nivo
Fermi level is the highest energy of occupied states in a solid at zero temperature. The Fermi level in conductors lies in the conduction band, in insulators it lies in the valence band, and in semiconductors it falls in the gap between the conduction band and the valence band. It was named after the Italian physicst Enrico Fermi (1901 - 1954).
collision theory → teorija sudara
Collision theory is theory that explains how chemical reactions take place and why rates of reaction alter. For a reaction to occur the reactant particles must collide. Only a certain fraction of the total collisions cause chemical change; these are called successful collisions. The successful collisions have sufficient energy (activation energy) at the moment of impact to break the existing bonds and form new bonds, resulting in the products of the reaction. Increasing the concentration of the reactants and raising the temperature bring about more collisions and therefore more successful collisions, increasing the rate of reaction.
Curie → Curie
Maria Sklodowska-Curie (1867-1934) Polish-born French chemist who went to Paris in 1891. She married the physicist Pierre Curie (1859-1906) in 1985 and soon began work on seeking radioactive elements other than uranium in pitchblende (to account for its unexpectedly high radioactivity). By 1898 she had discovered radium and polonium although it took her years to purify them. In 1903 the Curies shared the Nobel Prize for physics with Henri Becquerel, who had discovered radioactivity.
Citing this page:
Generalic, Eni. "Zakon o očuvanju energije." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table