metabolism → metabolizam
Metabolism is a sum of all chemical and physiological processes by which the body builds and maintains itself. It is a process of building the body’s molecular structures from nutrients (anabolism) and breaking them down for energy (catabolism).
molar enthalpy of evaporation → molarna entalpija isparavanja
Molar enthalpy of evaporation (Δl gH) is a change of enthalpy during evaporation divided by molarity of a liquid, and is equal to the heat energy spent when the evaporation is conducted under constant pressure, Δl gH=Q.
molar enthalpy of melting → molarna entalpija taljenja
Molar enthalpy of melting (Δs lH) is a change of enthalpy during melting divided by the molarity of a solid matter, and is equal to the energy used when melting is conducted under constant pressure.
open system → otvoreni sustav
Open system is a system which can exchange matter and energy with the environment.
electronegativity → elektronegativnost
Electronegativity is a parameter originally introduced by L. Pauling which describes, on a relative basis, the power of an atom to attract electrons. For example, in hydrogen chloride, the chlorine atom is more electronegative than the hydrogen and the molecule is polar, with a negative charge on the chlorine atom.
There are various ways of assigning values for the electronegativity of an element. Pauling electronegativities are based on bond dissociation energies using a scale in which fluorine, the most electronegative element, has the value 4 and francium, the lowest electronegative element, has the value 0.7.
enthalpy → entalpija
Enthalpy (H) is a thermodynamic property of a system defined by
where U is the internal energy of the system, p its pressure, and V its volume. J.W. Gibbs put the concept of an ensemble forward in 1902. In a chemical reaction carried out in the atmosphere the pressure remains constant and the enthalpy of reaction (ΔH), is equal to
For an exothermic reaction ΔH is taken to be negative.
entropy → entropija
Entropy (S) is a measure of the unavailability of a system’s energy to do work; in a closed system, an increase in entropy is accompanied by a decrease in energy availability. When a system undergoes a reversible change the entropy (S) changes by an amount equal to the energy (Q) transferred to the system by heat divided by the thermodynamic temperature (T) at which this occurs.
All real processes are to a certain extent irreversible changes and in any closed system an irreversible change is always accompanied by an increase in entropy.
Pauling scale → Paulingova skala
Pauling scale is a numerical scale of electronegativities based on bond-energy calculations for different elements joined by covalent bonds. Electronegativity is the power of an atom when in a molecule to attract eletrons to itself. Fluorine is the most electronegative element with a Pauling electronegativity value of 4.
perfect crystal → savršeni kristal
Perfect crystal is a crystal with no defects or impurities made of completely identical repeating subunits. Further, a perfect crystal has only one possible arrangement of subunits, with every subunit making exactly the same contribution to the total energy of the crystal.
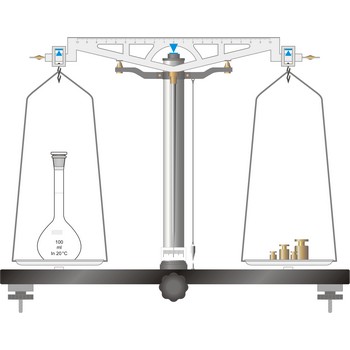
equal-arm balance → vaga s jednakim krakovima
The simplest type of balance, the equal-arm balance, is an application of a first class lever. The beam of the balance is supported on a central knife-edge, usually of agate, which rests upon a plane agate plate. The point of support is called the fulcrum. Two pans of equal weight are suspended from the beam, one at each end, at points equidistant from the fulcrum. A long pointer attached at right angles to the beam at the fulcrum indicates zero on a scale when the beam is at rest parallel to a level surface.
To prevent the knife-edge from becoming dull under the weight of the beam and pans the balance is equipped with a special device called an arrest. The arrest is operated by means of milled knob underneath the base plate in the middle and in front of the balance (sometimes the arrest knob is at one side of the balance).
The object to be weighed is placed on one pan, and standard weights are added to the other until the balance of the beam is established again. When not in use and during loading or unloading of the pans, the balance should be arrested.
Citing this page:
Generalic, Eni. "Zakon o očuvanju energije." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table