chemical balance → kemijska ravnoteža
Chemical balance is a degree of reversible reaction in a closed system, when the forward and backward reaction happen at same rates and their effects annul each other, while the concentration of reactants and products stays the same.
chemical cleaning → kemijsko čišćenje
Chemical cleaning is a removal of fatty and other impurities combined with it from fabrics with the help of organic solvents.
chemical engineering → kemijsko inženjerstvo
Chemical engineering is the branch of engineering that is concerned with the design, construction and operation of the plants and machinery used in industrial chemical processes.
chemical glass → kemijsko staklo
Chemical glass is a special, resilient glass used in production of chemical vessels; it is composed of quartz and boron, barium, zinc and aluminium oxides.
chemical property → kemijsko svojstvo
Chemical property is a property observed when a substance undergoes a transformation into one or more new substances. Measurement of a chemical property involves a chemical change. For example, determining the flammability of petrol involves burning it, producing carbon dioxide and water.
chemical compound formula → formula kemijskog spoja
Chemical elements are represented by their symbols, and chemical compounds are represented by a group of symbols of those elements from which the compound is composed. That group of symbols, which shows which atoms and in which number relation they are present in certain compound is called a chemical compound formula.
In a formula chemical symbols show which element is present in a certain compound, and its index shows how much of that element there is in a certain compound. From sulphuric acid formula H2SO4 we can see that one molecule of sulphuric acid consists of two atoms of hydrogen, one atom of sulphur and four atoms of oxygen.
dynamic equilibrium → dinamička ravnoteža
Dynamic equilibrium is established when two opposing processes are occurring at precisely the same rate, so that there is no apparent change in the system over long periods of time.
chemical weapons → kemijsko oružje
The Chemical Weapons Convention, article 2, paragraph 1 defines chemical weapons thus:
Chemical weapons means the following, together or separately:
(a) Toxic chemicals and their precursors, except where intended for purposes not prohibited under this Convention, as long as the types and quantities are consistent with such purposes;
(b) Munitions and devices, specifically designed to cause death or other harm through the toxic properties of those toxic chemicals specified in subparagraph (a), which would be released as a result of the employment of such munitions and devices;
(c) Any equipment specifically designed for use directly in connection with the employment of munitions and devices specified in subparagraph (b).
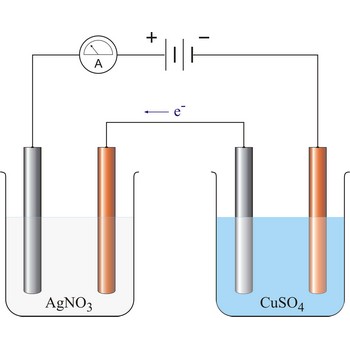
Faraday’s laws of electrolysis → Faradayevi zakoni elektrolize
Faraday’s laws of electrolysis are two laws found by British chemist and physicist Michael Faraday (1791-1867) in his experiments on electrolysis:
1. The quantity of matter extracted on the electrode is proportional to the quantity of charge (Q = I·t) which has flown in electrolysis time.
where z = number of electrons changed in reaction and F = Faraday’s constant which equals 96 487 C mol-1.
2. The masses of the elements liberated by the same quantity of electricity are directly proportional to their chemical equivalents.
96 487 C will discharge 1 mol Ag and 1/2 mol Cu. The relevant half reactions are:
thermodynamic equilibrium → termodinmička ravnoteža
Thermodynamic equilibrium is a system equilibrium in which energy that it gains from its surroundings is exactly balanced by the energy that it loses, no matter how much time is allowed to pass.
Citing this page:
Generalic, Eni. "Zakon o kemijskoj ravnoteži." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table