dielectric constant → dielektrična konstanta
Dielectric constant or permittivity (ε) is an index of the ability of a substance to attenuate the transmission of an electrostatic force from one charged body to another. The lower the value, the greater the attenuation. The standard measurement apparatus utilises a vacuum whose dielectric constant is 1. In reference to this, various materials interposed between the charged terminal have the following value at 20 °C:
| vacuum | 1 |
| air | 1.00058 |
| glass | 3 |
| benzene | 2.3 |
| acetic acid | 6.2 |
| ammonia | 15.5 |
| ethanol | 25 |
| glycerol | 56 |
| water | 81 |
The exceptionally high value for water accounts for its unique behaviour as a solvent and in electrolytic solutions. Dielectric constant values decrease as the temperature rises.
differential thermal analysis → diferencijalna termalna analiza
Differential thermal analysis (DTA) is a technique that is often used to analyze materials that react or decompose at higher temperatures. The difference in temperature between the sample and an inert reference material is monitored as both are heated in a furnace. Phase transitions and chemical reactions taking place in the sample on heating cause the temperature difference to become larger, at temperatures that are characteristic of the sample.
dipole → dipol
Dipole is a pair of separated opposite electric charges. Electric dipole is an assemblage of atoms or subatomic particles having equal electric charges of opposite sign separated by a finite distance. In the case of HCl, the electrons are attracted towards the more electronegative chlorine atom.
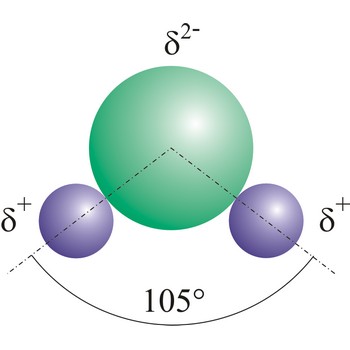
dipole molecule → dipolna molekula
Dipole molecules are created when mutual electronic pair at covalent bond is asymmetrical. If different atoms are bonded by a covalent bond, which can have different electron affinity, then the the atom with greater electron affinity will attract the electron pairs more strongly. In this way an asymmetrical distribution of negative charge appears in a molecule, so one part of the molecule becomes relatively negatively (the one closer to the electron pair) and the other becomes relatively positively charged.
neutron number → neutronski broj
Neutron number (N) is a characteristic property of a specific isotope of an element, equal to the number of neutrons in the nucleus.
nucleophile → nukleofil
Nucleophiles are negatively charged or bear a partial negative charge. Examples are lone pairs or a hydroxide ion.
paramagnetism → paramagnetizam
Paramagnetism is a type of magnetism characterised by a positive magnetic susceptibility, so that the material becomes weakly magnetised in the direction of an external field. The magnetisation disappears when the field in removed.
dipole moment → dipolni moment
Electric dipole moment (μ) is a product of the positive charge and the distance between the charges. Dipole moments are often stated in debyes; The SI unit is the coulomb metre. In a diatomic molecule, such as HCl, the dipole moment is a measure of the polar nature of the bond; i.e. the extent to which the average electron charges are displaced towards one atom (in the case of HCl, the electrons are attracted towards the more electronegative chlorine atom). In a polyatomic molecule, the dipole moment is the vector sum of the dipole moments of the individual bonds. In a symmetrical molecule, such as tetrafluoromethane (CF4) there is no overall dipole moment, although the individual C-F bonds are polar.
electric field → električno polje
If a small amount of charge experience a force, there is an electric field in the vicinity. Electric field E is defined in terms of electrostatic force F that would be exerted on positive test charge qp placed in the field:
SI unit for electric field is N C-1, or V m-1.
The electric field due to a point charge q at distance r from it given by:
where ε0 is permittivity constant, and is εo=8.85×10-12 C2 N-1 m-2.
Citing this page:
Generalic, Eni. "Wire gauge chart." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table