cathode → katoda
Cathode is a negative electrode of an electrolytic cell to which positively charged ions (cations) migrate when a current is passed as in electroplating baths.
In a primary or secondary cell (battery or accumulator) the cathode is the electrode that spontaneously becomes negative during discharge, and form which therefore electrons emerge.
In vacuum electronic devices electrons are emitted by the cathode and flow to the anode.
extensive property → ekstenzivno svojstvo
Extensive property is a property that changes when the amount of matter in a sample changes. Examples are mass, volume, length, and charge.
farad → farad
Farad (F) is the SI derived unit of electric capacitance. The farad is the capacitance of an electric capacitor between the two plates of which there appears a difference of electric potential of one volt when it is charged by a quantity of electricity equal to one coulomb (F = C/V). The unit was named after the British scientist M. Faraday (1791-1867).
Faradaic reaction → Faradejska reakcija
Faradaic reaction is a heterogeneous charge-transfer reaction occurring at the surface of an electrode.
Faraday constant → Faradayeva konstanta
Faraday constant (F) is the electric charge of 1 mol of singly charged positive ions.
where NA is Avogadro’s constant (6.022×1023 mol-1) and e is the elementary charge (1.602×10-19 C).
cathode ray → katodna zraka
Cathode ray is a negatively charged beam that emanates from the cathode of a discharge tube. Cathode rays are streams of electrons.
cetane number → cetanski broj
Cetane number is a measure of the ignition quality of diesel fuel. It denotes the volume fraction of cetane (C16H34) in a combustible mixture (containing cetane and 1-methylnapthalene) whose ignition characteristics match those of the diesel fuel being tested. Cetane is a collection of un-branched open chain alkane molecule that ignites very easily under compression, so it was assigned a cetane number of 100, while alpha-methyl naphthalene was assigned a cetane number of 0.
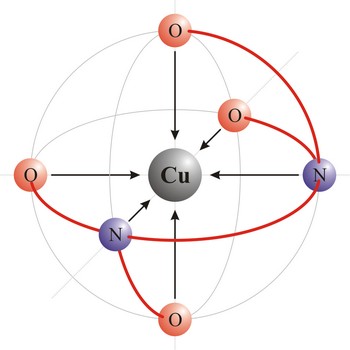
chelate → kelat
Chelate is a compound characterized by the presence of bonds from two or more bonding sites within the same ligand to a central metal atom. For example, copper complexes with EDTA to form a chelate. Chelate complexes are more stable than complexes with the corresponding monodentate ligands.
fluorescence → fluorescencija
Fluorescence is a luminescence phenomenon in which electron returns to it's ground state almost instantaneously (less than 10-8 second), and in which emission from a luminescent substance ceases when the exciting source is removed. Fluorescence is characterized by radiation emission in all directions.
Citing this page:
Generalic, Eni. "Wire gauge chart." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table