antiparticle → antičestica
Antiparticle is a particle having the same mass as a given elementary particle and a charge equal in magnitude but opposite in sign.
alkali metal → alkalijski metal
Alkali metal is a term that refers to six elements: lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). These elements make up group 1 of the periodic table of elements. They all form singly charged positive ions, and are extremely reactive. They react violently with water, forming hydroxides and releasing hydrogen gas and heat. Caesium and francium are the most reactive and lithium is the least.
arginine → arginin
Arginine is an electrically charged amino acids with basic side chains. It is one of the least frequent amino acids. As a group the charged amino acids are important for making proteins soluble. These residues are generally located on the surface of the protein. Arginine is well designed to bind the phosphate anion, and is often found in the active centers of proteins that bind phosphorylated substrates. As a cation, arginine, as well as lysine, plays a role in maintaining the overall charge balance of a protein. Although arginine is considered an essential amino acid (it must be obtained through the diet), this is true only during the juvenile period in humans.
- Abbreviations: Arg, R
- IUPAC name: 2-amino-5-(diaminomethylideneamino)pentanoic acid
- Molecular formula: C6H14N4O2
- Molecular weight: 174.20 g/mol
aspartic acid → asparaginska kiselina
Aspartic acid is an electrically charged amino acids with acidic side chains. As a group the charged amino acids are relatively abundant and are generally located on the surface of the protein. Aspartic acid and glutamic acid play important roles as general acids in enzyme active centers, as well as in maintaining the solubility and ionic character of proteins. Aspartic acid (sometimes referred to as asparate depending on pH) is non-essential in mammals, being produced from oxaloacetate by transamination.
- Abbreviations: Asp, D
- IUPAC name: 2-aminobutanedioic acid
- Molecular formula: C4H7NO4
- Molecular weight: 133.10 g/mol
atomic absorption spectroscopy → atomska apsorpcijska spektroskopija
Atomic absorption spectroscopy (AAS) An analytical technique in which a sample is vapourised and the nonexcited atoms absorb electromagnetic radiation at characteristic wavelengths.
atomic number → atomski broj
Atomic number (Z) is a characteristic property of an element, equal to the number of protons in the nucleus. The atomic number and the element symbol are two alternate ways to label an element. In nuclide symbols, the atomic number is a leading subscript; for example, in 2He.
atomic spectroscopy → atomska spektroskopija
Atomic spectroscopy is an expensive analytical method which uses absorption (AAS), emission (AES) and fluorescent (AFS) characteristics of the analyte.
bathymetry → batimetrija
Bathymetry (from the Greek word bathus, deep) is the science of measuring the depths of the oceans, seas, lakes, etc. or the topographic maps of the sea floor that result from such measurements. Bathymetric charts are designed to present accurate, measurable description and visual presentation of the submerged terrain.
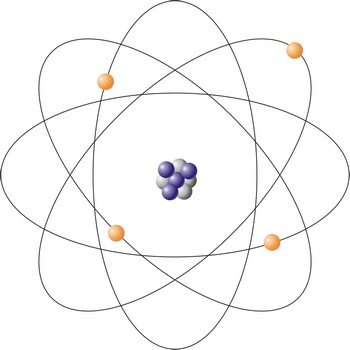
atom → atom
Atom is an atom is the smallest particle of an element that retains the chemical properties of the element. Rutherford-Bohr’s model represents the atom as a positively charged core of a size around 10-14 m composed of protons (positive particles) and neutrons (neutral particles) around which negatively charged electrons circle. The number of protons and electrons are equal, so the atom is an electrically a neutral particle. Diameter of the atom is about 10-10 m.
Citing this page:
Generalic, Eni. "Wire gauge chart." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table