volumetry → volumetrija
Volumetry consists of adding an equivalent amount of a reagent of exactly known concentration to the analyte. From stechiometrical proportion and added volume of reagent the quantity of matter in question can be calculated.
acid-base titration → kiselo-bazna titracija
Acid-base titration is an analytical technique in volumetric analysis, where an acid of known concentration is used to neutralise a known volume of a base, and the observed volume of the acid required is used to determine the unknown concentration of the base. An acid-base indicator is used to determine the end-point of the titration.
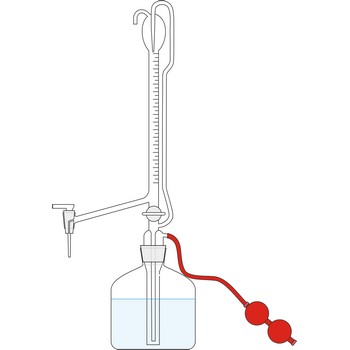
automatic burette → automatska bireta
Automatic burette is used for series of tests. It is connected with a bottle which contains the titration solution. The air is pumped into the bottle by a small rubber air pump, created the pressure in the bottle which the rises the solution to the top of burette. When the the burette is full, the valve is released, the pressure in the bottle falls and the burette automatically sets itself to zero. Work with automatic burettes is by far faster and the consumption of standard solution is smaller.
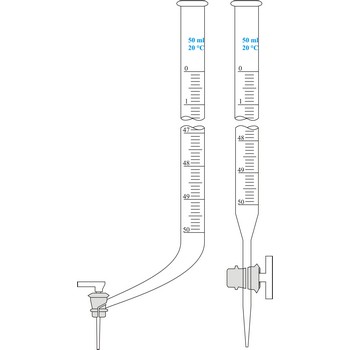
burette → bireta
Burette is a graded glass pipe which on its lower side has a glass faucet by which it can drop a precise quantity of liquid. Inner diameter of a burette must be equal in its whole length, because the accuracy of volume measurement depends upon that. Burettes are primarily used in volumetric analysis for titration with standard solution reagent. Most often Schellbach’s burette is used, graded on 50 mL with division of scale on 0.1 mL. Every burette is calibrated on discharge. For serial determining automatic burettes are used.
chlorosity → klorocitet
Chlorosity is the quantity determined by volumetric methods and is defined in the same manner as chlorinity except that the sample unit is 1 L of sea water rather than 1 kg of sea water weighed in vacuo.
conductometry → konduktometrija
Conductometry is a volumetric analytic method in which the end of titration (equivalent point) is defined by an electric conductivity appliance.
complexometry → kompleksometrija
Complexometry is a volumetric analytic method which is based on titration of metal ion solutions with a substance that, combined with metal ions yields complex compounds, e.g. EDTA. The stoichiometric ratio of EDTA-metal in complexometric analyses is always 1:1, whatever the valency of the metal
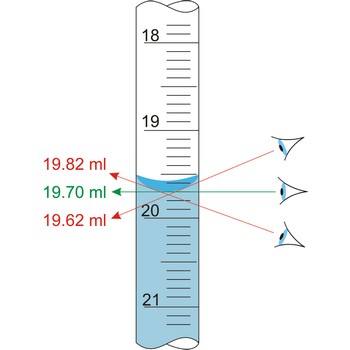
parallax → paralaksa
Parallax is a deceptive change of the position of an object which is observed while the position of the observer changes. Position of eye at all volumetric vessels must be at the same level as the meniscus. If not, the parallax will cause an error while reading the position of the meniscus of a liquid in a burette. It will be a positive mistake if the eye is lower, and negative if the eye is higher than the meniscus plane.
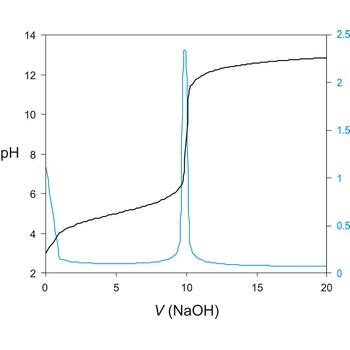
potentiometric titration → potenciometrijska titracija
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
Citing this page:
Generalic, Eni. "Volumetrija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table