polarography → polarografija
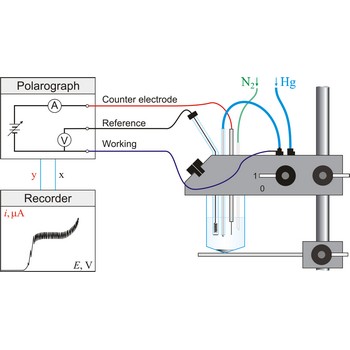
Polarography is a volumetric technique which is based on a diffusion controlled analyte travel to the surface of dropping mercury electrode (DME). The surface of the working electrode (dropping mercury electrode) is constantly renewed under dropping conditions and, thus, the conditions under which reaction takes place are readily reproducible. Depolarisation potential enables identification of ions present in the solution, and by measuring the diffusion current their concentration is calculated. Polarography was discovered in 1922 by the Czech chemist Jaroslav Heyrovský (1890-1967).
potentiometric titration → potenciometrijska titracija
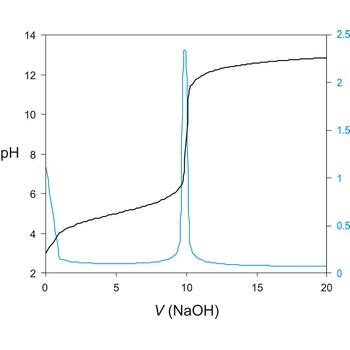
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
referent electrode → referentna elektroda
Referent electrode is an electrode whose potential is known and completely independent of analyte concentration. Mostly used referent electrodes are calomel and silver/silver chloride electrode.
Table: Dependence of referent electrodes potentials on KCl concentration
| Potential vs. SHE / V | |||||
| calomel electrode | Ag/AgCl electrode | ||||
| t / °C | 0.1 mol dm-3 | 3.5 mol dm-3 | sat. solution | 3.5 mol dm-3 | sat. solution |
| 15 | 0.3362 | 0.254 | 0.2511 | 0.212 | 0.209 |
| 20 | 0.3359 | 0.252 | 0.2479 | 0.208 | 0.204 |
| 25 | 0.3356 | 0.250 | 0.2444 | 0.205 | 0.199 |
| 30 | 0.3351 | 0.248 | 0.2411 | 0.201 | 0.194 |
| 35 | 0.3344 | 0.246 | 0.2376 | 0.197 | 0.189 |
reversible reaction → reverzibilna reakcija
Reversible reaction is a chemical reaction that can proceed in both the forward and backward directions. When reversible reactions reach equilibrium the forward and reverse reactions are still happening but at the same rate, so the concentrations of reactants and products do not change. A reversible reaction is denoted by a double arrow pointing both directions in a chemical equation.
rhenium → renij
Rhenium was discovered by Walter Noddack, Ida Tacke and Otto Berg (Germany) in 1925. The origin of the name comes from the Latin word Rhenus meaning river Rhine. It is rare and costly, dense, silvery-white metal. Tarnishes in moist air. Resists corrosion and oxidation. Dissolves in nitric and sulfuric acids. Has a very high melting point. Rhenium is found in small amounts in gadolinite and molybdenite. Mixed with tungsten or platinum to make filaments for mass spectrographs. Its main value is as a trace alloying agent for hardening metal components that are subjected to continuous frictional forces.
saturated solution → zasićena otopina
Saturated solution is a solution that holds the maximum possible amount of dissolved material. When saturated, the rate of dissolving solid and that of recrystallisation solid are the same, and a condition of equilibrium is reached. The amount of material in solution varies with temperature; cold solutions can hold less dissolved solid material than hot solutions. Gases are more soluble in cold liquids than in hot liquids.
seawater → more
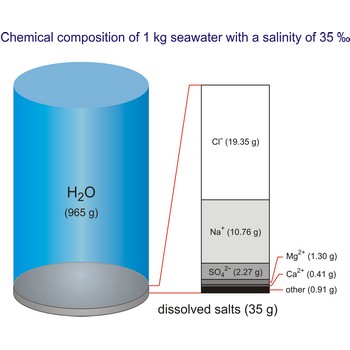
Seawater is a complex mixture of 96.5 % water, 3.5 % salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases. The world's oceans cover nearly 71 % (361 840 000 km2) of the Earth's surface (510 100 000 km2), with an average depth of 3 682.2 m.
The density of seawater is higher than that of fresh water because of its higher salinity. Seawater's freezing point is lower than that of pure water and its boiling point is higher. The average salinity of the ocean is 35 ‰, which means that for every kilograms of water, there are 35 g of salt. The relative abundance of the major salts in seawater are constant regardless of the ocean. Only six elements and compounds comprise about 99 % of sea salts: chlorine (Cl-), sodium (Na+), sulfur (SO42-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+).
salinity → salinitet
Salinity (S) is a measure of the quantity of dissolved salts in seawater. It is formally defined as the total amount of dissolved solids in seawater in parts per thousand (‰) by weight when all the carbonate has been converted to oxide, the bromide and iodide to chloride, and all organic matter is completely oxidized.
Chlorinity is the oldest of the salinity measures considered and is still a corner-stone in the study of dissolved material in seawater. Based on the principle of constant relative proportions it provides a measure of the total amount of dissolved material in seawater in terms of the concentration of halides. The relationship between chlorinity (Cl) and salinity as set forth in Knudsen’s tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
Practical Salinity (SP) was introduced as a replacement for Chlorinity. Practical Salinity is is relatively easy to measure using standard conductometers, measurements are more precise and less time consuming than measurements of Chlorinity and accurate measurements can even be made in situ. Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution).
Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". For most purposes one can assume that the psu and the ‰, are synonymous.
The global average salinity of ocean waters is about 35 ‰, that is, about 35 g of solid substances are dissolved in 1 kg of seawater.
second-order reactions → reakcije drugog reda
Second-order reaction is a reaction with a rate law that is proportional to either the concentration of a reactant squared, or the product of concentrations of two reactants.
For a general unimolecular reaction,
The reaction rate expression for a second order reaction is
If assumed that the concentration of reactant A is [A]o at t=0 and [A] at time T, the variables in the rate equation and integrate can be separated. The integrated rate law for a second-order reaction can be easily shown to be
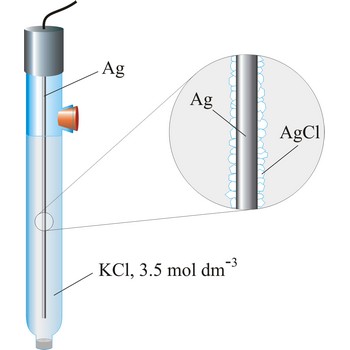
silver/silver-chloride electrode → srebro/srebrov klorid elektroda
Silver/silver-chloride electrode is by far the most common reference type used today because it is simple, inexpensive, very stable and non-toxic. It is mainly used with saturated potassium chloride electrolyte, but can be used with lower concentrations such as 3.5 mol dm-3 or 1 mol dm-3 potassium chloride. Silver/silver-chloride electrode is a referent electrode based on the following halfreaction
| Potential vs. SHE / V | ||
|---|---|---|
| t / °C | 3.5 mol dm-3 | sat. solution |
| 15 | 0.212 | 0.209 |
| 20 | 0.208 | 0.204 |
| 25 | 0.205 | 0.199 |
| 30 | 0.201 | 0.194 |
| 35 | 0.197 | 0.189 |
Citing this page:
Generalic, Eni. "Volumenska koncentracija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table