complexometry → kompleksometrija
Complexometry is a volumetric analytic method which is based on titration of metal ion solutions with a substance that, combined with metal ions yields complex compounds, e.g. EDTA. The stoichiometric ratio of EDTA-metal in complexometric analyses is always 1:1, whatever the valency of the metal
concentration → koncentracija
1. Group of four quantities characterizing the composition of a mixture with respect to the volume of the mixture (mass, amount, volume and number concentration).
2. Short form for amount (of substance) concentration (substance concentration in clinical chemistry).
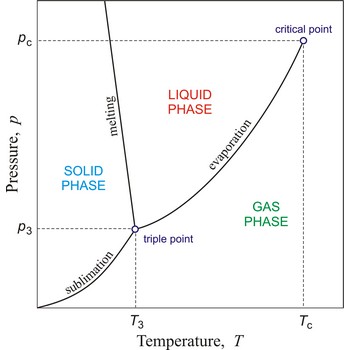
critical pressure → kritični tlak
Critical pressure is the pressure of a fluid in its critical point; i.e. when it is at its critical temperature and critical volume.
density → gustoća
In the most common usage, density (ρ) is mass density or mass per unit volume. In Si units it is measured in kg m-3. More commonly, densities are given in kg dm-3.
More generally, it is the amount of some quantity (mass, charge, energy, etc.) divided by a length, area, or volume.
Relative density is the ratio of the density of a substance to the density of some reference substance. For liquids or solids, it is the ratio of the density (usually at 20 °C) to the density of water at 4 °C. This quantity was formerly called specific gravity.
enthalpy → entalpija
Enthalpy (H) is a thermodynamic property of a system defined by
where U is the internal energy of the system, p its pressure, and V its volume. J.W. Gibbs put the concept of an ensemble forward in 1902. In a chemical reaction carried out in the atmosphere the pressure remains constant and the enthalpy of reaction (ΔH), is equal to
For an exothermic reaction ΔH is taken to be negative.
experiment → eksperiment
Experiment is direct observation under controlled conditions. Most experiments involve carefully changing one variable and observing the effect on another variable (for example, changing temperature of a water sample and recording the change volume that results).
face-centered cubic lattice → plošno centrirana kubična rešetka
Face-centered cubic lattice (fcc or cubic-F), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centers of each face of the unit cell. It has unit cell vectors a =b =c and interaxial angles α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the fcc structures the spheres fill 74 % of the volume. The number of atoms in a unit cell is four (8×1/8 + 6×1/2 = 4). There are 26 metals that have the fcc lattice.
gas → plin
Gas is a state of matter, in which the mollecules move freely and consequently the entire mass tends to expand indefinitely, occupying the total volume of any vessel into which it is introduced. Gases follow, within considerable degree of fidelity, certain laws relating their conditions of pressure, volume and temperature. Gases mix freely with each other, and they can be liquefied through compression or temperature reduction.
gas liquefying → ukapljivanje plinova
In order to achieve transition of a gas into liquid state it is necessary to lower its temperature, or decrease its volume, or increase its pressure. Above the critical temperature it is impossible to liquefy a gas. When liquefying a gas by Linde’s procedure, dampening or Joule-Thomson’s effect is used. First, the compressed air from the compressor is cooled with cooling water, the cooled air expands at a lower pressure in the dampening valve at which it cooled. The cooled air now returns to the compressor, cooling down the expanding air. By repeating this process the air is cooled enough to transit to the liquid state.
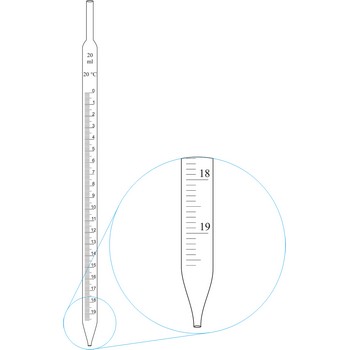
graduated pipette → graduirana pipeta
Graduated pipettes (Mohr pipette) have a scale divided into units of one and of 1/10th of a millilitre. Because of their wide necks it is less accurate than the volumetric pipette. They are used when taking volume of solutions in which accuracy does not have to be very high. By sucking in (with mouth, propipette or a water pump) the liquid is pulled in a little bit above the mark and the opening of the pipet is closed with a forefingertip. Outer wall of pipet is wiped and, with a slight forefinger loosening, the liquid is released until it reaches the mark 0. A pipette is emptied out by lifting the forefinger off and letting the liquid flow out of the pipette freely.
Citing this page:
Generalic, Eni. "Volumen." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table