Results 1–3 of 3 for voltametrija
cyclic voltammetry → ciklička voltametrija
Cyclic voltammetry (CV) is an electrochemical measuring technique used for the determination of the kinetics and mechanism of electrode reactions. The potential of the working electrode is controlled (typically with a potentiostat) and the current flowing through the electrode is measured. It is a linear-weep voltammetry with the scan continued in the reverse direction at the end of the first scan. This cycle can be repeated a number of times, and is used for corrosion studies.
voltametry → voltametrija
Voltametry is a common name for a large group of instrumental techniques which are based on measuring the electric current formed by a continuous potential shifting on the electrodes.
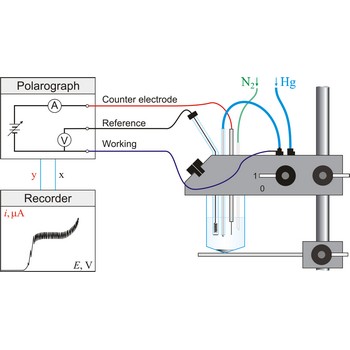
polarography → polarografija
Polarography is a volumetric technique which is based on a diffusion controlled analyte travel to the surface of dropping mercury electrode (DME). The surface of the working electrode (dropping mercury electrode) is constantly renewed under dropping conditions and, thus, the conditions under which reaction takes place are readily reproducible. Depolarisation potential enables identification of ions present in the solution, and by measuring the diffusion current their concentration is calculated. Polarography was discovered in 1922 by the Czech chemist Jaroslav Heyrovský (1890-1967).
Citing this page:
Generalic, Eni. "Voltametrija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table