metal → metal
Metals are materials in which the highest occupied energy band (conduction band) is only partially filled with electrons.
Their physical properties generally include:
- They are good conductors of heat and electricity. The electrical conductivity of metals generally decreases with temperature.
- They are malleable and ductile in their solid state.
- They show metallic lustre.
- They are opaque.
- They have high density.
- They are solids (except mercury)
- They have a crystal structure in which each atom is surrounded by eight to twelve near neighbours
Their chemical properties generally are:
- They have one to four valence electrons.
- They have low ionisation potentials; they readily lose electrons.
- They are good reducing agents.
- They have hydroxides which are bases or amphoteric.
- They are electropositive.
Metallic characteristics of the elements decrease and non-metallic characteristics increase with the increase of valence electrons. Also metallic characteristics increase with the number of electron shells. Therefore, there is no sharp dividing line between the metals and non-metals.
Of the 114 elements now known, only 17 show primarily non-metallic characteristics, 7 others are metalloids, and 89 may be classed as metals.
mutarotation → mutarotacija
Mutarotation is the change in optical rotation accompanying epimerization. In carbohydrate chemistry this term usually refers to epimerization at the hemiacetal carbon atom. In general α- and β-form are stable solids, but in solution they rapidly equilibrate. For example, D-glucose exists in an equilibrium mixture of 36 % α-D-glucopyranose and 64 % β-D-glucopyranose, with only a tiny fraction in the open-chain form. The equilibration occurs via the ring opening of the cyclic sugar at the anomeric center with the acyclic form as the intermediate. Mutarotation was discovered by French chemist Augustin-Pierre Dubrunfaut (1797-1881) in 1846.
non-metal → nemetal
Non-metals are defined as elements that are not metals.
Their physical properties generally include:
- They are poor conductors.
- They are brittle, not ductile in their solid state.
- They show no metallic lustre.
- They may be transparent or translucent.
- They have low density.
- They form molecules which consists of atoms covalently bonded; the noble gases are monoatomic.
Their chemical properties are generally:
- They usually have four to eight valence electrons.
- They have high electron affinities (except the noble gases)
- They are good oxidising agents (except the noble gases)
- They have hydroxides which are acidic (except the noble gases)
- They are electronegative.
nonpolar molecule → nepolarna molekula
Nonpolar molecule is a molecule which has no separation of charge, so no positive or negative poles are formed. For example, the Cl2 molecule has no polar bonds (molecule with one type of atom), CH4 is a non-polar molecule (due to its symmetry). Nonpolar molecules do not dissolve in water as they cannot form hydrogen bonds (thus are hydrophobic) but do dissolve in lipids or fats (lipophilic).
organic → organski
1. Organic refers to any chemical compound based on carbon (C) with the exception of some of the simple compounds of carbon, such as carbon dioxide, which are frequently classified as inorganic compounds. Additional elements that are commonly found in organic compounds are hydrogen (H), nitrogen (N), oxygen (O), phosphorus (P) and sulfur (S).
2. Organic or organically-grown foods are grown or raised without synthetic fertilizers, pesticides, growth stimulators, or antibiotics and other drugs. Pests are controlled by cultivation techniques and the use of pesticides derived from natural sources and the use of natural fertilizers. In addition, organically grown foods must also be stored without the use of chemicals such as artificial additives and preservatives, and without food irradiation.
osmium → osmij
Osmium was discovered by Smithson Tennant (England) in 1803. The origin of the name comes from the Greek word osme meaning smell. It is hard fine black powder or hard, lustrous, blue-white metal. Unaffected by air, water and acids. Characteristic acrid, chlorine like odour due to tetroxide compound. Osmium tetroxide highly toxic. Osmium is obtained from the same ores as platinum. Used to tip gold pen points, instrument pivots, to make electric light filaments. Used for high temperature alloys and pressure bearings. Very hard and resists corrosion better than any other.
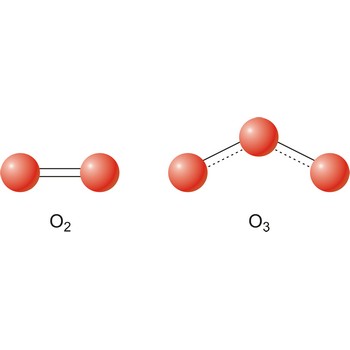
oxygen → kisik
Oxygen was discovered by Joseph Priestley (England) in 1774. The origin of the name comes from the Greek words oxy genes meaning acid and forming (acid former). It is colourless, odourless gas; pale blue liquid. Extremely reactive. Forms oxides with nearly all other elements except noble gases. It is the most abundant element in the earth’s crust and makes up almost 21 % of the atmosphere. Oxygen is obtained primarily from liquid air by fractional distillation. Small amounts are made in the laboratory by electrolysis of water. Used in steel making, welding and supporting life. Naturally occurring ozone (O3) in the upper atmosphere shields the earth from ultraviolet radiation.
palladium → paladij
Palladium was discovered by William Hyde Wollaston (England) in 1803. Named after the asteroid Pallas which was discovered at about the same time and from the Greek name Pallas, goddess of wisdom. It is soft, malleable, ductile, silvery-white metal. Resists corrosion; dissolves in oxidizing acids. Absorbs hydrogen. Metal dust is combustible. Palladium is obtained with platinum, nickel, copper and mercury ores. Used as a substitute for silver in dental items and jewellery. The pure metal is used as the delicate mainsprings in analog wristwatches. Also used in surgical instruments and as catalyst.
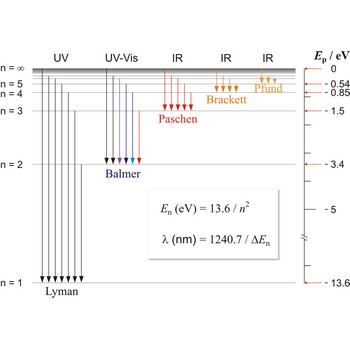
Paschen series → Paschenova serija
Paschen series are the series of lines in the spectrum of the hydrogen atom which corresponds to transitions between the state with principal quantum number n = 3 and successive higher states.
Citing this page:
Generalic, Eni. "Vodik." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table