gold → zlato
Gold has been known since ancient times. The origin of the name comes from the Latin word aurum meaning gold. It is soft, malleable, bright yellow metal. Unaffected by air, water, alkalis and most acids. Gold is found in veins in the crust, with copper ore and native. Used in electronics, jewellery and coins. It is a good reflector of infrared radiation, so a thin film of gold is applied to the glass of skyscrapers to reduce internal heating from sunlight.
hydrogen → vodik
Hydrogen was discovered by Sir Henry Cavendish (England) in 1766. The origin of the name comes from the Greek words hydro and genes meaning water and generate. It is colourless, odourless gas, burns and forms explosive mixtures in air. Reacts violently with oxidants. Hydrogen is the most abundant element in the universe. Commercial quantities of hydrogen are produced by reacting superheated steam with methane or carbon. In lab work from reaction of metals with acid solutions or electrolysis. Most hydrogen is used in the production of ammonia and in metal refining. Also used as fuel in rockets. Its two heavier isotopes (deuterium and tritium) used respectively for nuclear fusion.
ionisation → ionizacija
Ionisation is the process of producing ions. Certain molecules ionise in a solution; for example, acids ionise when dissolved in water.
Electron transfer also causes ionisation in certain reactions, for example sodium and chlorine react by transfer of a valence electron from the sodium atom to the chlorine atom to form the ions that constitute a sodium chloride crystal.
latex → lateks
Latex, also known as rubber or natural latex, is a milky fluid derived from the rubber tree. The latex is a colloid of caoutchouc (25-35%) dispersed in water (60-75%), which forms rubber by coagulation. The term is also applied to artificial emulsions of natural or artificial rubber, or of certain synthetic resins (such as polyvinyl acetate or polyvinyl chloride). It is used in a wide variety of consumer products, including rubber gloves, tubing, condoms, rubber bands, etc.
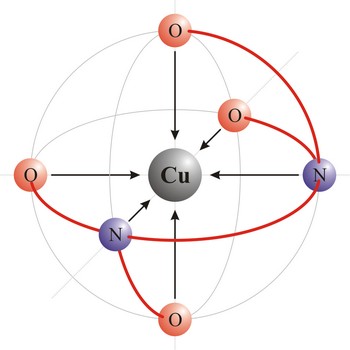
ligand → ligand
Ligand is an ion (F-, Cl-, Br-, I-, S2-, CN-, NCS-, OH-, NH2-) or molecule (NH3, H2O, NO, CO) that donates a pair of electrons to a metal atom or ion in forming a coordination complex. The main way of classifying ligands is by the number of points at which they are attached to, or bound to, the metal center. This is the denticity. Ligands with one potential donor atom are monodentate. Polydentate ligand is a ligand that is attached to a central metal ion by bonds from two or more donor atoms. Ligands with more than one potential donor atom are known as ambidentate, such as the thiocyanate ion, NCS-, which can bind to the metal center with either the nitrogen or sulphur atoms. Chelating ligands are those polydentate ligands which can form a ring including the metal atom.
Knudsen burette → Knudsenova bireta
Knudsen's automatic bulb-burette, developed by the Danish physicist Martin Knudsen (1871-1949), is designed in a way that even routine field analysis in a boat laboratory would provide highly accurate measurements. The burette is filled with a mixture of silver nitrate from reservoir R, located above the burette, by opening the A valve. When the solution crosses the three-way C valve the A valve is closed preventing further solution flow in to the burette. Any extra solution is caught in the W bowl. Turn the C valve, which marks the zero on the scale, in order to allow atmospheric air to enter the burette. Since most open-ocean samples lie in a relatively small chlorinity range, the burette is designed so that much of its capacity is in the bulb (B). This allows the titration to be quick (by quickly releasing contents from the B area) and reduces the error that occurs from the slow drainage along the inner wall of the burette.
Each millimeter is divided in to twenty parts (double millimeter division of the Knudsen burette) which allows for highly accurate measurements (the scale is read up to a precision of 0.005 mL). From 0 to 16 the burette isn't divided, that usually starts from 16 and goes until 20.5 or 21.5. A single double millimeter on a Knudsen burette scale corresponds to one permille of chloride in the seawater sample. This burette can be used for titration of water from all of the oceans and seas, with the exemptions being areas with very low salinity (e.g. the Baltic Sea) and river estuaries which require the use of normal burettes.
mineral → mineral
Minerals are compounds in which metals can be found in nature. Metals in nature can appear as:
| autochthonous | Au, Cu, Pt, Ag, Pd, Hg, Ir |
| oxides | Fe, Al, Sn, Cr, Mn, W, Cu |
| sulphides | Cu, Pb, Zn, Ni, Ag, Co, Sb, Hg, Mo, Cd, Bi |
| carbonates | Fe, Zn, Cu, Mg, Mn, Pb |
| silicates | Ni, Cu, Zn, Mn |
| chlorides | Ag, Cu, Mg, Na, K |
| sulphates | Ca, Ba, Sr, Cu |
polymer → polimer
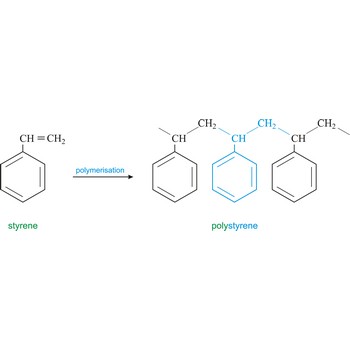
Polymer is a substance composed of molecules of high relative molecular mass (molecular weight), the structure of which essentially comprises the multiple repetition of units derived, actually or conceptually, from molecules of low relative molecular mass (monomers). In most cases the number of monomers is quite large and is often not precisely known. A single molecule of a polymer is called a macromolecule. Polystyrene is light solid material obtained by polymerisation of styrene (vinyl benzene).
polystyrene → polistiren
Polystyrene is a vinyl polymer. Structurally, it is a long hydrocarbon chain, with a phenyl group attached to every other carbon atom. Polystyrene is produced by free radical vinyl polymerization, from the monomer styrene. Polystyrene or Styrofoam is used in the construction industry as insulating material and for production of containers.
practical salinity → praktični salinitet
Practical salinity SP is defined on the Practical Salinity Scale of 1978 (PSS-78) in terms of the conductivity ratio K15 which is the electrical conductivity of the sample at temperature t68 = 15 °C and pressure equal to one standard atmosphere, divided by the conductivity of a standard potassium chloride (KCl) solution at the same temperature and pressure. The mass fraction of KCl in the standard solution is 0.0324356 (32.4356 g of KCl in 1 kg of solution). When K15 = 1, the Practical Salinity P S is by definition 35. The conductivity of that reference solution is C(35,1568,0) = 42.914 mS/cm = 4.2914 S/m (Siemens per meter). Note that Practical Salinity is a unit-less quantity. Though sometimes convenient, it is technically incorrect to quote Practical Salinity in "psu". When K15 is not unity, SP and K15 are related by the PSS-78 equation
At a temperature of t68 = 15 °C, Rt is simply K15 and Practical Salinity SP can be determined from the above equation. For temperatures other than t68 = 15 °C, Practical Salinity SP is given by the following function of Rt (k = 0.0162)
Citing this page:
Generalic, Eni. "Vinil klorid." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table