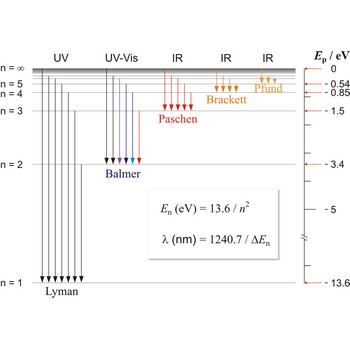
Paschen series → Paschenova serija
Paschen series are the series of lines in the spectrum of the hydrogen atom which corresponds to transitions between the state with principal quantum number n = 3 and successive higher states.
periodic table of the elements → periodni sustav elemenata
Periodic table is a table of elements, written in sequence in the order of atomic number or atomic weight and arranged in horizontal rows (periods) and vertical columns (groups) to illustrate the occurrence of similarities in the properties of the elements as a periodic function of the sequence. The original form was proposed by Dmitri Mendeleev (1834-1907) in 1869, using relative atomic masses.
phosphorescence → fosforescencija
Phosphorescence is emission of light from a substance exposed to radiation and persisting as an afterglow after the exciting energy has been removed. Unlike fluorescence, in which the absorbed energy is spontaneously emitted about 10-8 second after excitation, phosphorescence requires additional excitation to produce radiation and may last from about mili second to days or years, depending on the circumstances.
photoelectric effect → fotoelektrični efekt
Photoelectric effect is the complete absorption of a photon by a solid with the emission of an electron. The energy of a photon (hν) is
polonium → polonij
Polonium was discovered by Marie Curie (Poland) in 1898. Named for Poland, native country of Marie Curie. It is silvery-grey, extremely rare, radioactive metal. Soluble in dilute acids. Highly toxic. Severe radiotoxicity. Carcinogen. Polonium occurs in pitchblende. Produced by bombarding bismuth with neutrons. Used in industrial equipment that eliminates static electricity caused by such processes as rolling paper, wire and sheet metal.
Schrodinger equation → Schrodingerova jednadžba
Schrödinger equation is the basic equation of wave mechanics which, for systems not dependent on time, takes the form:
where Ψ is the wavefunction, V is the potential energy expressed as a function of the spatial coordinates, E its total energy, ![]() 2 is the Laplacian operator, h is Planck’s constant, and m is the mass.
2 is the Laplacian operator, h is Planck’s constant, and m is the mass.
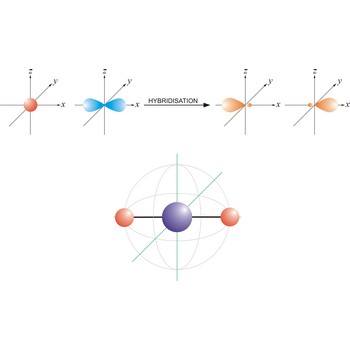
sp hybrid orbital → sp hibridna orbitala
An sp hybrid orbital is an orbital formed by the linear combination of one s and one p orbital of comparable energy (such 2s and 2p orbitals) on a same atom. The two sp hybrid orbitals are aligned in a straight line in opposite direction (bond angles are 180°). The remaining two p orbitals are at right angles to one another and to the line formed by the two sp orbitals.
sp2 hybrid orbital → sp2 hibridna orbitala
An sp2 hybrid orbital is an orbital formed by the linear combination of one s and two p orbitals of comparable energy (such 2s and 2p orbitals) on a same atom. The three sp2 hybrid orbitals lie in a plane with angle of 120°. The remaining p orbital remains unchanged and is perpendicular to the plane of the three sp2 orbitals.
solar cell → sunčeva ćelija
Solar cell, or photovoltaic cell, is a device that captures sunlight and transforms it directly to electricity. All solar cells make use of photovoltaic effect, so often they are called photovoltaic cells. Almost all solar cells are built from solid-state semiconducting materials, and in the vast majority of these the semiconductor is silicon.
The photovoltaic effect involves the generation of mobile charge carriers-electrons and positively charged holes-by the absorption of a photon of light. This pair of charge carriers is produced when an electron in the highest filled electronic band of a semiconductor (the valence band) absorbs a photon of sufficient energy to promote it into the empty energy band (the conduction band). The excitation process can be induced only by a photon with an energy corresponding to the width of the energy gap that separates the valence and the conduction band. The creation of an electron-hole pair can be converted into the generation of an electrical current in a semiconductor junction device, wherein a layer of semiconducting material lies back to back with a layer of either a different semiconductor or a metal. In most photovoltaic cells, the junction is p-n junction, in which p-doped and n-doped semiconductors are married together. At the interface of the two, the predominance of positively charged carriers (holes) in the p-doped material and of negatively charged carriers (electrons) in the n-doped material sets up an electric field, which falls off to either side of the junction across a space-charge region. When absorption of a photon in this region generates an electron-hole pair, these charge carriers are driven in opposite directions by the electric field, i.e. away from the interface and toward the top and bottom of the two-layer structure, where metal electrodes on these faces collect the current. The electrode on the top layer (through which light is absorbed) is divided into strips so as not to obscure the semiconducting layers below. In most widely used commercial solar cells, the p-doped and n-doped semiconductive layers are formed within a monolithic piece of crystalline silicon. Silicon is able to absorb sunlight at those wavelengths at which it is most intense-from the near-infrared region (wavelengths of around 1200 nm) to the violet (around 350 nm).
sp3 hybrid orbital → sp3 hibridna orbitala
An sp3 hybrid orbital is an orbital formed by the linear combination of one s and three p orbitals of comparable energy (such 2s and 2p orbitals) on a same atom. The four sp3 hybrid orbitals point toward the corners of a regular tetrahedron with the bond angle of 109.5°.
Citing this page:
Generalic, Eni. "Vertikalna energija ionizacije." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table