wavefunction → valna funkcija
Wavefunction (Ψ) is a mathematical function that gives the amplitude of a wave as a function of position (and sometimes as a function of time and/or electron spin). Wavefunctions are used in chemistry to represent the behaviour of electrons bound in atoms or molecules.
wavelength → valna duljina
Wavelength is the distance between two neighbouring peaks of the electromagnetic wave.
wave-particle duality → valno-čestična dualnost
Wave-particle duality is an observation that electrons, photons, and other very small entities behave like particles in some experiments and like waves in others.
wavenumber → valni broj
Wavenumber is the number of wave cycles per unit distance.
There are unfortunately two different definitions of the wavenumber.
Wavenumber, k, is most frequently defined as
with wavelength λ, phase velocity of wave vp, and angular frequency ω.
Less frequently it is defined simply as
One must be careful to note which definition is in use. Wavenumbers are used extensively in infrared spectroscopy, and usually have units of cm-1.
atomic orbital → atomska orbitala
Atomic orbital is a wave function that describes the behaviour of an electron in an atom.
Beer’s law → Beerov zakon
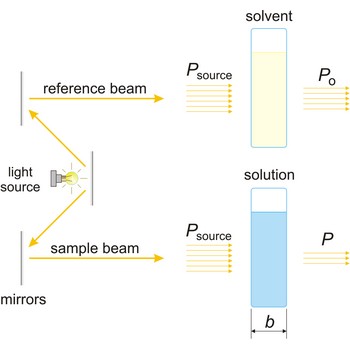
Beer’s law (or Beer-Lambert law) is the functional relationship between the quantity measured in an absorption method (A) and the quantity sought, the analyte concentration (c). As a consequence of interactions between the photons and absorbing particles, the power of the beam is attenuated from Po to P. Beer’s law can be written
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
blackbody radiation → zračenje crnog tijela
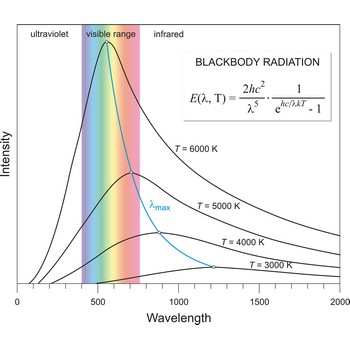
Blackbody radiation is the radiation emitted by a perfect blackbody, i.e., a body which absorbs all radiation incident on it and reflects none. The primary law governing blackbody radiation is the Planck Radiation Law, which governs the intensity of radiation emitted by unit surface area into a fixed direction (solid angle) from the blackbody as a function of wavelength for a fixed temperature. The Planck Law can be expressed through the following equation
where λ is the wavelength, h is Planck’s constant, c is the speed of light, k is the Boltzmann constant, and T is the temperature.
hybridization → hibridizacija
Hybridization is an internal linear combination of atomic orbitals, in which the wave functions of the atomic orbitals are added together to generate new hybrid wave functions. The new orbitals which are formed are hybrids of the originals and have properties (shape, size and energy) that are somewhere in between.
Schrodinger equation → Schrodingerova jednadžba
Schrödinger equation is the basic equation of wave mechanics which, for systems not dependent on time, takes the form:
where Ψ is the wavefunction, V is the potential energy expressed as a function of the spatial coordinates, E its total energy, ![]() 2 is the Laplacian operator, h is Planck’s constant, and m is the mass.
2 is the Laplacian operator, h is Planck’s constant, and m is the mass.
absorbance → apsorbancija
Absorbance (A) is a logarithm of the ratio of incident radiant power (Po) to transmitted radiant power (P) through a sample (excluding the effects on cell walls).
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
Citing this page:
Generalic, Eni. "Valna funkcija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table