photoelectric effect → fotoelektrični efekt
Photoelectric effect is the complete absorption of a photon by a solid with the emission of an electron. The energy of a photon (hν) is
photomultiplier → fotomultiplikator
Photomultiplier (photomultiplier tube or PMT) is a very versatile and sensitive detector of radiant energy in the ultraviolet, visible, and near infrared regions of the electromagnetic spectrum. A typical photomultiplier tube consists of a photoemissive cathode (photocathode) followed by focusing electrodes, an electron multiplier (dynode) and an electron collector (anode) in a vacuum tube.
plasma → plazma
Plasma is a highly ionised gas in which the charge of the electrons is balanced by the charge of the positive ions, so that the system as a whole is electrically neutral. Plasmas are created by exposing gases at low pressure to an electric or electromagnetic field. In semiconductor processing, plasmas are used for etching and thin film deposition (the excited state of the gas makes it very reactive). In everyday life plasmas are used to give light in fluorescent light bulbs, neon lamps, and blue insect traps.
polarography → polarografija
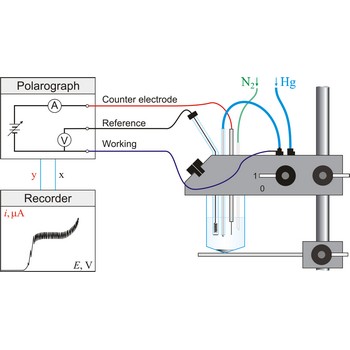
Polarography is a volumetric technique which is based on a diffusion controlled analyte travel to the surface of dropping mercury electrode (DME). The surface of the working electrode (dropping mercury electrode) is constantly renewed under dropping conditions and, thus, the conditions under which reaction takes place are readily reproducible. Depolarisation potential enables identification of ions present in the solution, and by measuring the diffusion current their concentration is calculated. Polarography was discovered in 1922 by the Czech chemist Jaroslav Heyrovský (1890-1967).
polydentant ligand → polidentantni liganad
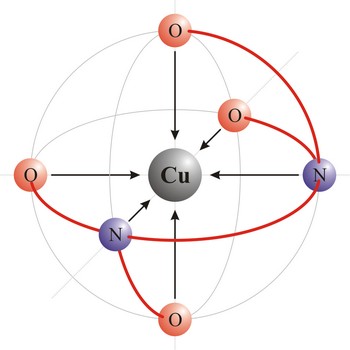
Polydentant ligands contain more co-ordination points (can give more electron pairs) and they form complex ringlike structures (celate complexes) by replacing two or more monodentant ligands. That kind of ligand is EDTA which has 6 co-ordinational points and with metals it creates complexes, always in 1:1 ratio.
potentiometric titration → potenciometrijska titracija
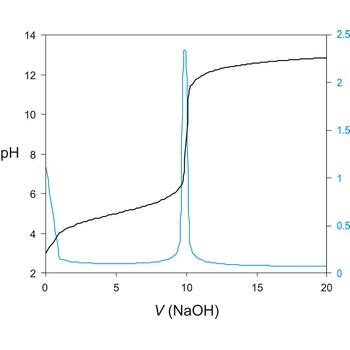
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
precision balance → tehnička vaga
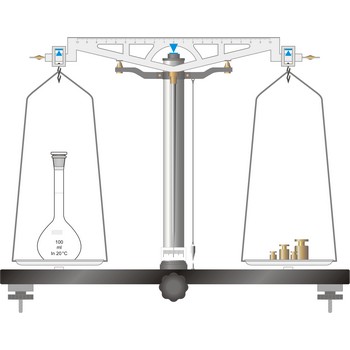
Precision balances typically display results from three to one decimal places (0.001 g up to 0.1 g). The readability precision balances are reduced when compared to analytical balances but, precision balances accommodate higher capacities (up to several kilograms). In its traditional form, it consists of a pivoted horizontal lever of equal length arms, called the beam, with a weighing pan, also called scale, suspended from each arm.
In electronic top pan, or toploader balances, mass is determined not by mechanical deflection but by electronically controlled compensation of an electric force. The signal generated enables the mass to be read from a digital display. The mass of the empty container can be stored in the balance’s computer memory and automatically deducted from the mass of the container plus its contents.
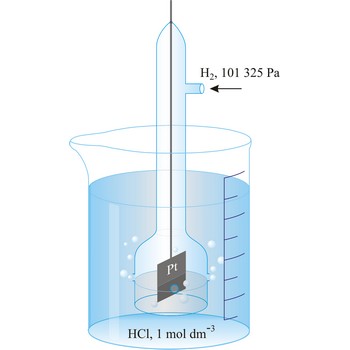
redox potential → redoks potencijal
Redox potential is the potential of a reversible oxidation-reduction electrode measured with respect to a reference electrode, corrected to the hydrogen electrode, in a given electrolyte.
redox reaction → redoks-reakcija
Redox reaction is an oxidation-reduction reaction is a reaction in which one or more electrons are transferred. When an atom, ion, or molecule loses one or more electrons, it is oxidised. When an atom, ion, or molecule gains one or more electrons, it is reduced.
reducing agent → redukcijsko sredstvo
Reducing agent may be defined in various ways, depending upon the context in which the phrase is used. In broad terms it is often taken to mean a chemical which can act as an electron donor. Thus, in the reaction:
the zinc is being reduced (gaining electrons) by reaction with the iron cations; the Fe2+ in this instance is acting as a reducing agent.
Citing this page:
Generalic, Eni. "Valentni elektron." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table