valence shell → valentna ljuska
Valence shell is the shell corresponding to the highest value of principal quantum number in the atom. The valence electrons in this shell are on average farther from the nucleus than other electrons. They are often directly involved in chemical reaction.
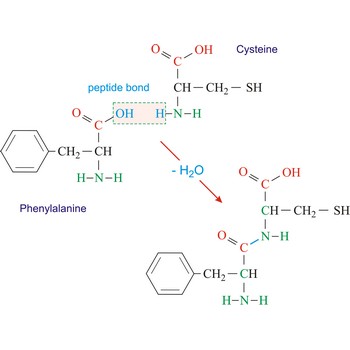
peptide bond → peptidna veza
Peptide bond emerges when two amino acid join in a way that the carbon atom from one connects with the nitrogen atom from the other (creating a C-N bond).
valence electron → valentni elektron
Valence electrons are electrons that can be actively involved in a chemical change, usually electrons in the outermost (valent) shell. For example, sodium’s ground state electron configuration is 1s2 2s2 2p6 3s1; the 3s electron is the only valence electron in the atom. Germanium (Ge) has the ground state electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2; the 4s and 4p electrons are the valence electrons.
theoretical yield → teoretski prinos
Theoretical yield is the maximum quantity of a product that could be formed in a chemical reaction if all the limiting reactants reacted to form products (distinguished from actual yield).
theories of catalysis → teorije katalize
Theories of catalysis explain the influence of the catalysts upon the rate of a reaction by describing the detailed mechanism by which the catalyst is involved in the steps of the chemical reaction.
Lewis, Gilbert N. → Lewis, Gilbert N.
Gilbert Newton Lewis (1875-1946) is an American chemist whose theory of the electron pair fostered understanding of the covalent bond and extended the concept of acids and bases.
molecular shape → oblik molekule
Molecular shape is the three dimensional arrangement of atoms in space around a central atom. The molecular formula of a substance does not give an indication of its shape. For example, CO2 is a linear molecule, but SO2 is angular.
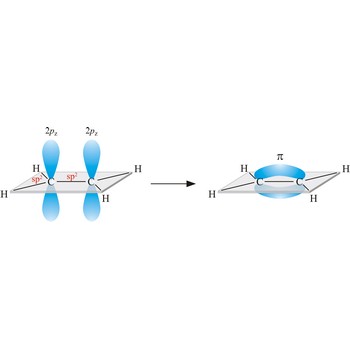
The three-dimensional shapes of many small molecules can be predicted by applying the valence shell electron pair repulsion theory (VSEPR). When atoms combine to form molecules, pairs of valence electrons arrange themselves as far from each other as possible. Another way to characterize molecular shape is in terms of hybrid orbitals.
non-metal → nemetal
Non-metals are defined as elements that are not metals.
Their physical properties generally include:
- They are poor conductors.
- They are brittle, not ductile in their solid state.
- They show no metallic lustre.
- They may be transparent or translucent.
- They have low density.
- They form molecules which consists of atoms covalently bonded; the noble gases are monoatomic.
Their chemical properties are generally:
- They usually have four to eight valence electrons.
- They have high electron affinities (except the noble gases)
- They are good oxidising agents (except the noble gases)
- They have hydroxides which are acidic (except the noble gases)
- They are electronegative.
Citing this page:
Generalic, Eni. "Valence bond theory." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table