seawater → more
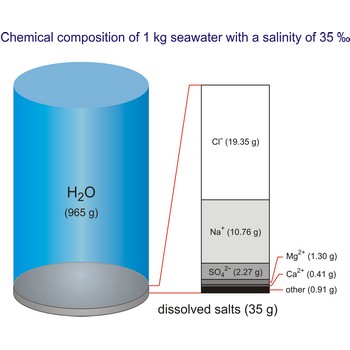
Seawater is a complex mixture of 96.5 % water, 3.5 % salts, and smaller amounts of other substances, including dissolved inorganic and organic materials, particulates, and a few atmospheric gases. The world's oceans cover nearly 71 % (361 840 000 km2) of the Earth's surface (510 100 000 km2), with an average depth of 3 682.2 m.
The density of seawater is higher than that of fresh water because of its higher salinity. Seawater's freezing point is lower than that of pure water and its boiling point is higher. The average salinity of the ocean is 35 ‰, which means that for every kilograms of water, there are 35 g of salt. The relative abundance of the major salts in seawater are constant regardless of the ocean. Only six elements and compounds comprise about 99 % of sea salts: chlorine (Cl-), sodium (Na+), sulfur (SO42-), magnesium (Mg2+), calcium (Ca2+), and potassium (K+).
smoke → dim
Smoke is a fine suspension of solid particles in a gas. In general smoke particles range downward from about 5 μm in diameter to less than 01 μm in diameter. Smoke generally refers to a visible mixture of products given off by the incomplete combustion of an organic substance such as wood, coal, fuel oil etc. This airborne mixture general contains small particles (dusts) of carbon, hydrocarbons, ash etc. as well as vapors such as carbon monoxide, carbon dioxide, and water vapor.
solubility → topljivost
Solubility is the maximum amount of solute that dissolves in a given quantity of solvent at a specific temperature. Generally, for a solid in a liquid, solubility increases with temperature; for a gas, solubility decreases. Common measures of solubility include the mass of solute per unit mass of solution (mass fraction), mole fraction of solute, molality, molarity, and others.
standard hydrogen electrode → standardna vodikova elektroda
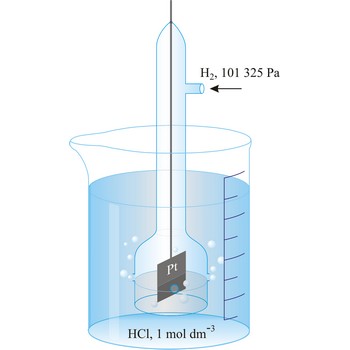
Standard hydrogen electrode is a system in which hydrogen ion and gaseous hydrogen are present in their standard states. The convention is to designate the cell so that the standard hydrogen electrode is written first.
The electrode is used as a reference (of zero) for the values of other standard electrode potentials.
state of matter → agregatno stanje
State of matter is one of the tree physical states in which matter can exist, i.e. solid, liquid or gas. Plasma is sometimes regarded as the fourth state of matter. By means of heating a solid substance will cross to liquid state at its melting point. If we heat up a liquid and beyond, at its boiling point it will cross to gaseous state - vapour.
supercritical fluid → superkritični fluid
Supercritical fluid is any substance above its critical temperature and critical pressure (see phase diagram). It shows unique properties that are different from those of either gases or liquids under standard conditions. A supercritical fluid has both the gaseous property of being able to penetrate anything, and the liquid property of being able to dissolve materials into their components. Solublity increases with increasing density (i.e. with increasing pressure). An example of this is naphthalene which is practically insoluble in low pressure carbon dioxide. At 100 bar the solubility is 10 g/L and at 200 bar it is 50 g/L. Rapid expansion of supercritical solutions leads to precipitation of a finely divided solid.
tear gas → suzavac
Tear gases is the common name for substances which, in low concentrations, cause pain in the eyes, flow of tears and difficulty in keeping the eyes open. Tear gases are used mainly in military exercises and in riot control, etc., but have also been used as a method of warfare. Irritating gases have been used in war since ancient times but it was not until after the Second World War that a more systematic search for effective substances was started. Among a long series of substances, three have become of greater importance than the others. These substances are chloroacetophenone (codename CN), orto-chlorobenzylidene-malononitrile (CS) and dibenz(b,f)-1,4-oxazepine (CR).
thermochemical equation → termokemijska jednadžba
Thermochemical equation is a compact equation representing a chemical reaction that describes both the stoichiometry and the energetics of the reaction. For example, the thermochemical equation
means When one mole of gaseous methane is burned in two moles of oxygen gas, one mole of carbon dioxide gas and 2 moles of steam are produced, and 2 220 kJ of heat are released.
van der Waals’ equation → van der Waalsova jednadžba
Van der Waals’ equation is an equation of state for real fluids which takes the form:
where p is pressure, Vm is molar volume, T is temperature, R is the molar gas constant, and a and b are characteristic parameters of the substance which describe the effect of attractive and repulsive intermolecular forces.
Citing this page:
Generalic, Eni. "Ukapljivanje plinova." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table