sand filtration → pješčana filtracija
Sand filtration is a frequently used and very robust method of removing suspended solids from water. The filtration medium consists of a multiple layer of sand with a variety in size and specific gravity. Sand filters can be supplied in different sizes and materials, both hand operated and fully automatically.
dissociation constant → konstanta disocijacije
Dissociation constant is a constant whose numerical value depends on the equilibrium between the undissociated and dissociated forms of a molecule. A higher value indicates greater dissociation.
The term dissociation is also applied to ionisation reactions of acids and bases in water. For example
which is often regarded as a straightforward dissociation into ions
The equilibrium constant of such a dissociation is called the acid dissociation constant or acidity constant, given by
The concentration of water [H2O] can be taken as constant.
Similarly, for a base, the equilibrium
is also a dissociation; with the base dissociation constant or basicity constant, given by
Ka (Kb) is a measure of the strength of the acid (base).
dysprosium → disprozij
Dysprosium was discovered by Paul Emile Lecoq de Boisbaudran (France) in 1886. The origin of the name comes from the Greek word dysprositos meaning hard to obtain. It is soft, lustrous, silvery metal. Reacts with oxygen. Reacts rapidly with water; dissolves in acids. Metal ignites and burns readily. Reductant. Dysprosium usually found with erbium, holmium and other rare earths in some minerals such as monazite sand. Dysprosium uses are limited to the experimental and esoteric. Some isotopes of dysprosium are effective absorbers of thermal neutrons and are being considered for use in the control rods in nuclear reactors.
solvation → solvatacija
Solvation is the process by which solvent molecules surround and interact with solute ions or molecules.
strong electrolyte → jaki elektrolit
Strong electrolytes are those electrolytes which in water solutions completely dissociate into their ions. They conduct electric current very well.
electrochemical cell → elektrokemijski članak
Electrochemical cell is a device that converts chemical energy into electrical energy or vice versa when a chemical reaction is occurring in the cell. It consist of two electronically conducting phases (e.g., solid or liquid metals, semiconductors, etc) connected by an ionically conducting phase (e.g. aqueous or non-aqueous solution, molten salt, ionically conducting solid). As an electric current passes, it must change from electronic current to ionic current and back to electronic current. These changes of conduction mode are always accompanied by oxidation/reduction reactions.
An essential feature of the electrochemical cell is that the simultaneously occurring oxidation-reduction reactions are spatially separated. E.g., in a spontaneous chemical reaction during the oxidation of hydrogen by oxygen to water, electrons are passed directly from the hydrogen to the oxygen.
In contrast, in the spontaneous electrochemical reaction in a galvanic cell the hydrogen is oxidised at the anode by transferring electrons to the anode and the oxygen is reduced at the cathode by accepting electrons from the cathode. The ions produced in the electrode reactions, in this case positive hydrogen ions and the negative hydroxyl (OH-) ions, will recombine in the solution to form the final product of the reaction: water. During this process the electrons are conducted from the anode to the cathode through an outside electric circuit where the electric current can drive a motor, light a light bulb, etc. The reaction can also be reversed: water can be decomposed into hydrogen and oxygen by the application of electrical power in an electrolytic cell.
electrochemical series → elektrokemijski niz
Electrochemical series is a series of chemical elements arranged in order of their standard electrode potentials. The hydrogen electrode
is taken as having zero electrode potential. An electrode potential is, by definition, a reduction potential.
Elements that have a greater tendency than hydrogen to lose electrons to their solution are taken as electropositive; those that gain electrons from their solution are below hydrogen in the series and are called electronegative.
The series shows the order in which metals replace one another from their salts; electropositive metals will replace hydrogen from acids.
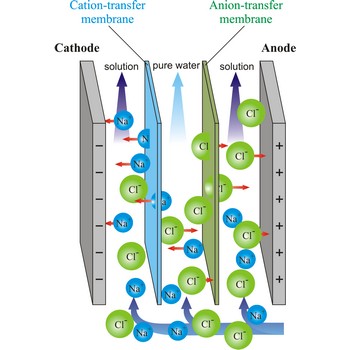
electrodialysis → elektrodijaliza
Electrodialysis is a procedure of dialysis accelerated with an electric field. Dialyser is divided into three sections. Solution flows through the middle section, between two semipermeable membranes alternately to positive ions and negative ions. An electrodes are placed in the neighbouring sections. Under the influence of electric field, positive ions will travel towards the cathode (the negative electrode), and negative ions towards the anode (the positive electrode), whereby travelling of ions through the membrane is accelerated. In this way, the feed water is separated into two streams: one of pure water and the other of more concentrated solution.
Citing this page:
Generalic, Eni. "Tvrdoća vode." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table