liquid crystal → tekući kristal
Liquid crystals or crystalline liquids are a physical state between crystals and melts. The liquid crystalline phase - the so-called mesophase - is formed at the melting point. The most important (usable) mesophases are nematic, cholesteric and smectic phase, having different molecular orientations.
Hesse’s law → Hessov zakon
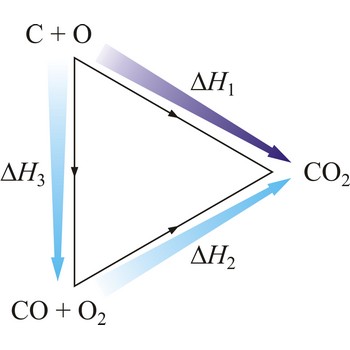
Hesse’s law says that reaction heat of some chemical change does not depend on the way in which the reaction is conducted, but only on starting and ending system state. Hesse’s law is also known as the law of constant heat summation. Hesse’s law is also known as the law of constant heat summation. The law was first put forward in 1840 by the Swiss-born Russian chemist Germain Henri Hess (1802-1850).
Hesse’s law can be used to obtain thermodynamic data that cannot be measured directly. For example, it is very difficult to control the oxidation of graphite to give pure CO. However, enthalpy for the oxidation of graphite to CO2 can easily be measured. So can the enthalpy of oxidation of CO to CO2. The application of Hess’s law enables us to estimate the enthalpy of formation of CO.
| C(s) + O2(g) →← CO2(g) | ΔrH1 = -393 kJ mol-1 |
| CO(g) + 1/2O2(g) →← CO2(g) | ΔrH2 = -283 kJ mol-1 |
| C(s) + 1/2O2(g) →← CO(g) | ΔrH3 = -110 kJ mol-1 |
The equation shows the standard enthalpy of formation of CO to be -110 kJ/mol.
hydration → hidratacija
Hydration is addition of water or the elements of water (i.e. H and OH) to a molecular entity. The term is also used in a more restricted sense for the process:
molar enthalpy of evaporation → molarna entalpija isparavanja
Molar enthalpy of evaporation (Δl gH) is a change of enthalpy during evaporation divided by molarity of a liquid, and is equal to the heat energy spent when the evaporation is conducted under constant pressure, Δl gH=Q.
molar quantity → molarna veličina
Molar quantity is often convenient to express an extensive quantity (e.g., volume, enthalpy, heat capacity, etc.) as the actual value divided by the amount of substance (number of moles). The resulting quantity is called molar volume, molar enthalpy, etc.
Peltier effect → Peltierov efekt
Peltier effect is the absorption or generation of heat (depending on the current direction) which occurs when an electric current is passed through a junction between two materials.
infrared radiation → infracrveno zračenje
Infrared radiation is an electromagnetic radiation within the area from 1.0 μm to 300 μm, and is responsible for the transmission of radiant heat.
lithosphere → litosfera
Lithosphere (from the Greek for rocky sphere) is rigid, rocky outer layer of the Earth, consisting of the crust and the solid outermost layer of the upper mantle. The distinguishing characteristic of the lithosphere is not its composition but its flow properties. It floats on the asthenosphere, which is the heat-softened layer of the mantle below the lithosphere.
The lithospheric is not one continuous piece but is broken into about a dozen major separate rigid blocks, or plates, which move independently relative to one another. This movement of lithospheric plates over the asthenosphere is described as plate tectonics. When an oceanic plate and a continental plate meet, the heavier oceanic plate (composed mostly of basalt, specific gravity about 3.0 or peridotite, specific gravity about 3.3) subducts under the lighter continental plate (composed mostly of granite, specific gravity about 2.7).
luminescence → luminiscencija
Luminescence (from Latin lumen, light) is the emission of electromagnetic radiation (UV, visible or IR) from atoms or molecules as a result of the transition of an electronically excited state to a lower energy state, usually the ground state. Luminescence can be divided into categories by duration (fluorescence or phosphorescence) or by the mechanism that creates the light (radioluminescence, electroluminescence, photoluminescence, thermoluminescence, triboluminescence, chemiluminescence, bioluminescence). The prefix identifies the energy source responsible for generating or releasing the light.
Phosphorescence is emission of light from a substance exposed to radiation and persisting as an afterglow after the source of excitation has been removed. Fluorescence, on the other hand, is an almost instantaneous effect, ending within about 10-8 second after excitation.
Citing this page:
Generalic, Eni. "Toplina taljenja." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table