law of conservation of mass → zakon o očuvanju mase
Law of conservation of mass states that no detectable gain or loss in mass occurs in chemical reactions. The state of a substance may change in a chemical reaction, for example, from a solid to a gas, but its total mass will not change. Note that the energy released (exothermic) or adsorbed (endothermic) in a chemical reaction is a result of energy transfer between atoms and their environment.
lead-acid battery → olovni akumulator
Lead-acid battery is a electrical storage device that uses a reversible chemical reaction to store energy. It was invented in 1859 by French physicist Gaston Planté. Lead-acid batteries are composed of a lead(IV) oxide cathode, a sponge metallic lead anode and a sulphuric acid solution electrolyte.
In charging, the electrical energy supplied to the battery is changed to chemical energy and stored. The chemical reaction during recharge is normally written:
In discharging, the chemical energy stored in the battery is changed to electrical energy. During discharge, lead sulfate (PbSO4) is formed on both the positive and negative plates. The chemical reaction during discharge is normally written:
Lead acid batteries are low cost, robust, tolerant to abuse, tried and tested. For higher power applications with intermittent loads however, they are generally too big and heavy and they suffer from a shorter cycle life.
reversible cell → povrativi članak
Reversible cell is an electrical cell the chemical action in which can be reversed by passing through it a current opposite in direction to that generated by the cell.
luminescence → luminiscencija
Luminescence (from Latin lumen, light) is the emission of electromagnetic radiation (UV, visible or IR) from atoms or molecules as a result of the transition of an electronically excited state to a lower energy state, usually the ground state. Luminescence can be divided into categories by duration (fluorescence or phosphorescence) or by the mechanism that creates the light (radioluminescence, electroluminescence, photoluminescence, thermoluminescence, triboluminescence, chemiluminescence, bioluminescence). The prefix identifies the energy source responsible for generating or releasing the light.
Phosphorescence is emission of light from a substance exposed to radiation and persisting as an afterglow after the source of excitation has been removed. Fluorescence, on the other hand, is an almost instantaneous effect, ending within about 10-8 second after excitation.
Schiff base → Schiffova baza
Schiff base is a class of compounds derived by the chemical reaction (condensation) of aldehydes or ketones with aromatic amines, for example
They were named after the German chemist Hugo Schiff (1834-1915).
spontaneously combustible → samozapaljivi materijal
Spontaneously combustible materials are materials that can ignite without an external source of heat. Heat sufficient to reach the ignition temperature may be generated by reaction with oxygen in the air, by the absorption of moisture, from heat generated during processing, or even from radioactive decay.
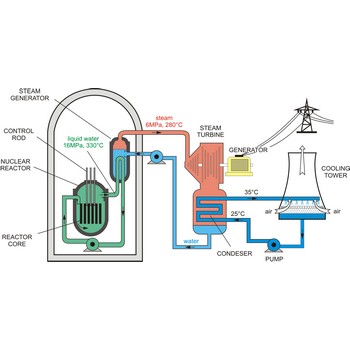
nuclear reactor → nuklearni reaktor
Nuclear reactor is an assembly of fissionable material (uranium-235 or plutonium-239) designed to produce a sustained and controllable chain reaction for the generation of electric power.
The essential components of a nuclear reactor are:
- The core, metal rods containing enough fissionable material to maintain a chain reaction at the necessary power level (as much as 50 t of uranium may be required).
- A source of neutrons to initiate the reaction (such as a mixture of polonium and beryllium)
- A moderator to reduce the energy of fast neutrons for more efficient fission (material such as graphite, beryllium, heavy water, and light water are used)
- A coolant to remove the fission-generated heat (water, sodium, helium, and nitrogen may be used)
- A control system such as rods of boron or cadmium that have high capture cross sections (to absorb neutrons)
- Adequate shielding, remote-control equipment, and appropriate instrumentation are essential for personnel safety and efficient operation.
octet rule → pravilo okteta
Octet rule states that the chemical properties of the elements repeat on a regular basis with increasing atomic mass, and that the chemical properties of each eight element are similar. Since the inert gases, with the exception of helium have eight electrons in their outer shells, this stable electronic configuration is called the octet rule. In chemical reactions atoms of elements tend to react in such a way as to achieve the electronic configuration of the inert gas nearest to them in the periodic table. There are a number of exceptions to the octet rule.
theories of catalysis → teorije katalize
Theories of catalysis explain the influence of the catalysts upon the rate of a reaction by describing the detailed mechanism by which the catalyst is involved in the steps of the chemical reaction.
potential energy → potencijalna energija
Potential energy (Ep) is the energy stored in a body or system as a consequence of its position, shape, or state (this includes gravitation energy, electrical energy, nuclear energy, and chemical energy). Gravitational potential energy is the energy associated with the state of separation between bodies that attracts each other via gravitational force. Elastic potential energy is the energy associated with the state of compression or extension of an elastic object. Thermal energy is associated with the random motions of atoms and molecules in a body.
Citing this page:
Generalic, Eni. "Toplina kemijske reakcije." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table