Hesse’s law → Hessov zakon
Hesse’s law says that reaction heat of some chemical change does not depend on the way in which the reaction is conducted, but only on starting and ending system state. Hesse’s law is also known as the law of constant heat summation. Hesse’s law is also known as the law of constant heat summation. The law was first put forward in 1840 by the Swiss-born Russian chemist Germain Henri Hess (1802-1850).
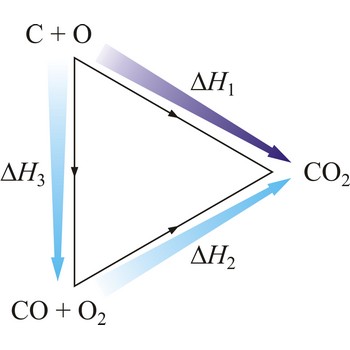
Hesse’s law can be used to obtain thermodynamic data that cannot be measured directly. For example, it is very difficult to control the oxidation of graphite to give pure CO. However, enthalpy for the oxidation of graphite to CO2 can easily be measured. So can the enthalpy of oxidation of CO to CO2. The application of Hess’s law enables us to estimate the enthalpy of formation of CO.
| C(s) + O2(g) →← CO2(g) | ΔrH1 = -393 kJ mol-1 |
| CO(g) + 1/2O2(g) →← CO2(g) | ΔrH2 = -283 kJ mol-1 |
| C(s) + 1/2O2(g) →← CO(g) | ΔrH3 = -110 kJ mol-1 |
The equation shows the standard enthalpy of formation of CO to be -110 kJ/mol.
lithosphere → litosfera
Lithosphere (from the Greek for rocky sphere) is rigid, rocky outer layer of the Earth, consisting of the crust and the solid outermost layer of the upper mantle. The distinguishing characteristic of the lithosphere is not its composition but its flow properties. It floats on the asthenosphere, which is the heat-softened layer of the mantle below the lithosphere.
The lithospheric is not one continuous piece but is broken into about a dozen major separate rigid blocks, or plates, which move independently relative to one another. This movement of lithospheric plates over the asthenosphere is described as plate tectonics. When an oceanic plate and a continental plate meet, the heavier oceanic plate (composed mostly of basalt, specific gravity about 3.0 or peridotite, specific gravity about 3.3) subducts under the lighter continental plate (composed mostly of granite, specific gravity about 2.7).
Rankine cycle → Rankineov ciklus
Rankine cycle is a thermodynamic cycle which can be used to calculate the ideal performance of a heat engine that uses a condensable vapour as the working fluid.
specific quantity → specifična veličina
Specific quantity is often convenient to express an extensive quantity (e.g., volume, enthalpy, heat capacity, etc.) as the actual value divided by mass. The resulting quantity is called specific volume, specific enthalpy, etc.
luminescence → luminiscencija
Luminescence (from Latin lumen, light) is the emission of electromagnetic radiation (UV, visible or IR) from atoms or molecules as a result of the transition of an electronically excited state to a lower energy state, usually the ground state. Luminescence can be divided into categories by duration (fluorescence or phosphorescence) or by the mechanism that creates the light (radioluminescence, electroluminescence, photoluminescence, thermoluminescence, triboluminescence, chemiluminescence, bioluminescence). The prefix identifies the energy source responsible for generating or releasing the light.
Phosphorescence is emission of light from a substance exposed to radiation and persisting as an afterglow after the source of excitation has been removed. Fluorescence, on the other hand, is an almost instantaneous effect, ending within about 10-8 second after excitation.
mercury → živa
Mercury has been known since ancient times. The origin of the name comes from the Latin word hydrargyrum meaning liquid silver. It is heavy, silver-white metal, liquid at ordinary temperatures. Stable in air and water. Unreactive with alkalis and most acids. Gives off poisonous vapour. Chronic cumulative effects. Mercury only rarely occurs free in nature. The chief ore is cinnabar or mercury sulfide (HgS). Used in thermometers, barometers and batteries. Also used in electrical switches and mercury-vapour lighting products.
metal → metal
Metals are materials in which the highest occupied energy band (conduction band) is only partially filled with electrons.
Their physical properties generally include:
- They are good conductors of heat and electricity. The electrical conductivity of metals generally decreases with temperature.
- They are malleable and ductile in their solid state.
- They show metallic lustre.
- They are opaque.
- They have high density.
- They are solids (except mercury)
- They have a crystal structure in which each atom is surrounded by eight to twelve near neighbours
Their chemical properties generally are:
- They have one to four valence electrons.
- They have low ionisation potentials; they readily lose electrons.
- They are good reducing agents.
- They have hydroxides which are bases or amphoteric.
- They are electropositive.
Metallic characteristics of the elements decrease and non-metallic characteristics increase with the increase of valence electrons. Also metallic characteristics increase with the number of electron shells. Therefore, there is no sharp dividing line between the metals and non-metals.
Of the 114 elements now known, only 17 show primarily non-metallic characteristics, 7 others are metalloids, and 89 may be classed as metals.
spontaneously combustible → samozapaljivi materijal
Spontaneously combustible materials are materials that can ignite without an external source of heat. Heat sufficient to reach the ignition temperature may be generated by reaction with oxygen in the air, by the absorption of moisture, from heat generated during processing, or even from radioactive decay.
system → sustav
System is the region under consideration, as distinguished from the rest of the universe (the environment). Systems may be separated from environments by boundaries that prevent the transfer of mass (a closed system), of heat (an adiabatic system), or of any energy (an isolated system). Systems that exchange mass with the environment are open systems.
Citing this page:
Generalic, Eni. "Toplina." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table