dialysis → dijaliza
Dialysis is a method by which large molecules (such as starch or protein) and small molecules (such as glucose or amino acids) may be separated in a solution by selective diffusion through a semipermeable membrane. Through this kind of membrane dissolved particles pass and colloid dimension particles fall behind. For example, if a mixed solution of starch and glucose is placed in a closed container made of a semipermeable substance (such as cellophane), which is then immersed in a beaker of water, the smaller glucose molecules will pass trough the membrane into the water, while the starch molecules remain behind.
dry cell → suhi članak
Dry cell or Leclanche cell is a primary cell having a zinc anode, a carbon (graphite) cathode surrounded by manganese dioxide, and a paste containing ammonium chloride as electrolyte. The electromotive force (emf) produced by a dry cell is 1.5 V. Dry cell is not reversible and therefore have a limited operating life. It is invented by the French engineer Georges Leclanché (1839.-1882.) in 1866.
termination → terminacija
Termination is the final step in a free radical mechanism that results in the stopping of the free radical reaction.
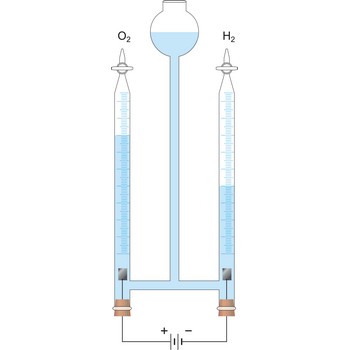
electrolysis → elektroliza
Electrolysis is the decomposition of a substance as a result of passing an electric current between two electrodes immersed in the sample.
electrolytes → elektroliti
Electrolytes are substances which, when melted or dissolved in water, conduct electric current. By melting or dissolving they are dissociated into electrically charged particles (ions) which are able to conduct electric current. By passing of electric current the transfer of matter occurs. Positively charged particles (cations) travel towards the negative pole (the cathode) and negatively charged particles (the anions) travel towards the positive pole (the anode). Liquid metals, in which the conduction is by free electrons, are not usually regarded as electrolytes. Solid conductors of ions, as in the sodium-sulphur cell, are also known as electrolytes. Depending upon how it conducts electric current, matter can be divided into strong electrolytes, weak electrolytes and nonconductors.
filtration → filtriranje
Filtration is a procedure in which liquids are separated from the precipitate by passing a suspension through the filter. The precipitate remains on the filter and through it the filtrate passes. Gaseous heterogeneous mixtures can also be filtrated.
Fischer-Tropsch process → Fischer-Tropschov postupak
Fischer-Tropsch process is an industrial method of making hydrocarbon fuels from carbon monoxide and hydrogen. The process was introduced in 1933. and used by Germany in World War II. to produce motor fuel. Hydrogen and carbon monoxide are mixed in the ratio 2:1 (water gas was used with added hydrogen) and passed at 200 °C over a nickel or cobalt catalyst. The resulting hydrocarbon mixture can be separated into a higher-boiling fraction for Diesel engines and a lower-boiling petrol fraction. The petrol fraction contains a high proportion of straight-chain hydrocarbons and has to be reformed for use in motor fuel. Alcohols, aldehydes, and ketones are also present. The process is also used in the manufacture of SNG from coal. It is named after the German chemist Franz Fischer (1852-1932) and the Czech Hans Tropsch (1839-1935).
thermodynamic equilibrium → termodinmička ravnoteža
Thermodynamic equilibrium is a system equilibrium in which energy that it gains from its surroundings is exactly balanced by the energy that it loses, no matter how much time is allowed to pass.
viscosity → viskozitet
Viscosity. (η) (coefficient of viscosity) is the resistance a liquid exhibits to flow. Experimentally, the frictional force between two liquid layers moving past each other is proportional to the area of the layers and the difference in flow speed between them.
white light → bijela svjetlost
White light is a mixture of lights of all colours. If white light is passed through a glass prism or an optical lattice, it is separated into several colours (the visible light spectrum).
Citing this page:
Generalic, Eni. "To pass final judgement中文." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table