Raman effect → Ramanov efekt
Raman effect is a type of scattering of electromagnetic radiation in which light suffers a change in frequency and a change in phase as it passes through a material medium. Named according to the Indian physicist C. V. Raman (1889-1970). The intensity of Raman scattering is about one-thousandth of that in Rayleigh scattering in liquids.
calendering → kalandriranje
Calendering is the process of forming materials to make a film/sheet by passing them through a series of hot rollers.
cathode → katoda
Cathode is a negative electrode of an electrolytic cell to which positively charged ions (cations) migrate when a current is passed as in electroplating baths.
In a primary or secondary cell (battery or accumulator) the cathode is the electrode that spontaneously becomes negative during discharge, and form which therefore electrons emerge.
In vacuum electronic devices electrons are emitted by the cathode and flow to the anode.
colloid → koloid
Colloids are systems in which there are two or more phases, with one (the dispersed phase) distributed in the other (the continuous phase). Moreover, at least one of the phases has small dimensions, in the range between 1 nm and 1 μm (10-9 m – 10-6 m). Dimension, rather than the nature of the material, is characteristic. In this size range, the surface area of the particle is large with respect to its volume so that unusual phenomena occur, e.g., the particles do not settle out of the suspension by gravity and are small enough to pass through filter membranes. Macromolecules (proteins and other high polymers) are at the lower limit of this range; the upper limit is usually taken to be the point at which the particles can be resolved in an optical microscope.
Colloidal particles may be gaseous, liquid, or solid, and occur in various types of suspensions:
Sols - dispersions of small solid particles in a liquid.
Emulsions - colloidal systems in which the dispersed and continuous phases are both liquids.
Gels - colloids in which both dispersed and continuous phases have a three-dimensional network throughout the material.
Aerosols - colloidal dispersions of liquid or solid particles in a gas.
Foams - dispersions of gases in liquids or solids.
reverse osmosis → reverzna osmoza
Reverse osmosis is the method used for obtaining freshwater from saltwater. The process uses a semi-permeable membrane through which pure water and not the salts will pass. The saltwater must be pressurised to approximately 25 bar, which makes it operationally expensive to produce large quantities of fresh water by this method.
reversible cell → povrativi članak
Reversible cell is an electrical cell the chemical action in which can be reversed by passing through it a current opposite in direction to that generated by the cell.
semi-permeable membrane → polupropusna membrana
Semi-permeable membrane is a membrane through which only some sorts of particles can pass, while others cannot.
size of the nucleus → veličina jezgre
Size of the nucleus was measured by Lord Rutherford using the scattering patterns of alpha particles passing through a gold foil. It is 10-15 m.
standing wave → stojni val
Standing waves occur when a travelling wave reflects from the fixed ends of a string, producing other waves moving in opposite direction. They are called standing waves because the energy in the string cannot move past the fixed ends, i.e. it stands in the string. In real strings, after some time, standing waves are eventually damped due to friction.
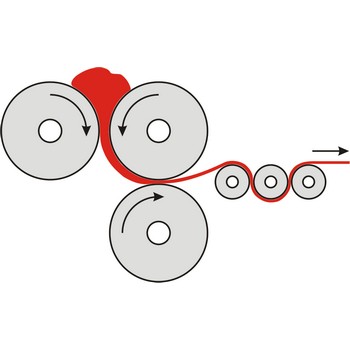
colloid mill → koloidni mlin
Colloid mills are machines used to grind aggregates into very fine particles or to apply very high shearing within a fluid to produce colloid suspensions or emulsions in which the particle sizes are less than 1 micrometer. One type of colloid mill is called a disc mill, in which a mixture of a solid and liquid (or two liquids) is passed between two discs a small distance apart, which rotate very rapidly relative to each other. Applications of colloid mills occur in food processing, in paint manufacture, and in the pharmaceutical industry.
Citing this page:
Generalic, Eni. "To pass final judgement中文." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table