Results 1–9 of 9 for termionska emisija
thermionic emission → termionska emisija
Thermionic emission is the boiling off of electrons from heated metals. It gives a source of electrons for cathode ray tubes.
atomic spectroscopy → atomska spektroskopija
Atomic spectroscopy is an expensive analytical method which uses absorption (AAS), emission (AES) and fluorescent (AFS) characteristics of the analyte.
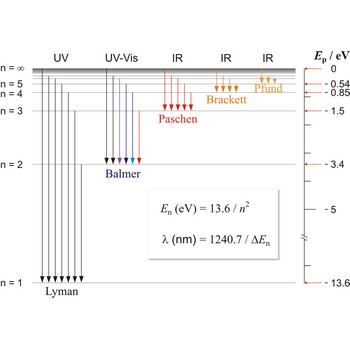
Balmer series → Balmerova serija
Balmer series, Balmer lines is a series of lines in the emission spectrum of hydrogen that involve transitions to the n=2 state from states with n>2.
Bohr atom → Bohrov atom
Bohr atom is a model of the atom that explains emission and absorption of radiation as transitions between stationary electronic states in which the electron orbits the nucleus at a definite distance. The Bohr model violates the Heisenberg uncertainty principle since it postulates definite paths and moment for electrons as they move around the nucleus. Modern theories usually use atomic orbitals to describe the behaviour of electrons in atoms.
fluorescence → fluorescencija
Fluorescence is a luminescence phenomenon in which electron returns to it's ground state almost instantaneously (less than 10-8 second), and in which emission from a luminescent substance ceases when the exciting source is removed. Fluorescence is characterized by radiation emission in all directions.
line spectrum → linijski spektar
Red-hot gases give line spectrum, i.e. is they emit electromagnetic rays of defined wavelengths. That kind of emission line of spectrum is characteristic of each chemical element.
luminescence → luminiscencija
Luminescence (from Latin lumen, light) is the emission of electromagnetic radiation (UV, visible or IR) from atoms or molecules as a result of the transition of an electronically excited state to a lower energy state, usually the ground state. Luminescence can be divided into categories by duration (fluorescence or phosphorescence) or by the mechanism that creates the light (radioluminescence, electroluminescence, photoluminescence, thermoluminescence, triboluminescence, chemiluminescence, bioluminescence). The prefix identifies the energy source responsible for generating or releasing the light.
Phosphorescence is emission of light from a substance exposed to radiation and persisting as an afterglow after the source of excitation has been removed. Fluorescence, on the other hand, is an almost instantaneous effect, ending within about 10-8 second after excitation.
polonium → polonij
Polonium was discovered by Marie Curie (Poland) in 1898. Named for Poland, native country of Marie Curie. It is silvery-grey, extremely rare, radioactive metal. Soluble in dilute acids. Highly toxic. Severe radiotoxicity. Carcinogen. Polonium occurs in pitchblende. Produced by bombarding bismuth with neutrons. Used in industrial equipment that eliminates static electricity caused by such processes as rolling paper, wire and sheet metal.
Citing this page:
Generalic, Eni. "Termionska emisija." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table