effective nuclear charge → efektivni naboj jezgre
Effective nuclear charge. (Zeff) is the nuclear charge experienced by an electron when other electrons are shielding the nucleus.
chelate → kelat
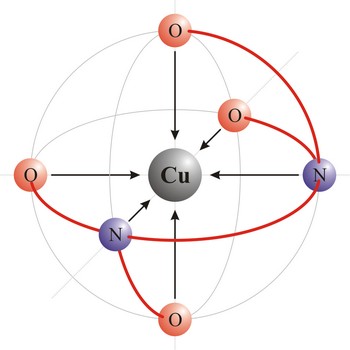
Chelate is a compound characterized by the presence of bonds from two or more bonding sites within the same ligand to a central metal atom. For example, copper complexes with EDTA to form a chelate. Chelate complexes are more stable than complexes with the corresponding monodentate ligands.
chiral molecule → kiralne molekule
Chiral molecule is a molecule which cannot be superimposed on its mirror image. A common example is an organic molecule containing a carbon atom to which four different atoms or groups are attached. Such molecules exhibit optical activity, i.e., they rotate the plane of a polarised light beam.
conformation → konformacija
Conformation is one of the very large numbers of possible spatial arrangements of atoms that can be interconverted by rotation about a single bond in a molecule. The conformation of a molecule is not fixed, though one or another shape may be more likely to occur. There are two extreme cases:
Staggered conformation (antiperiplanar) is a conformation about a carbon-carbon single bond in which the atoms on one carbon are as far apart as possible from the atoms on an adjacent carbon.
Eclipsed conformation (syn-periplanar) is a conformation about a carbon-carbon single bond in which the atoms on one carbon are as close as possible to the atoms on an adjacent carbon.
electron pair → elektronski par
Electron pair is two electrons within one orbital with opposite spins responsible for a chemical bond.
Fermi level → Fermijev nivo
Fermi level is the highest energy of occupied states in a solid at zero temperature. The Fermi level in conductors lies in the conduction band, in insulators it lies in the valence band, and in semiconductors it falls in the gap between the conduction band and the valence band. It was named after the Italian physicst Enrico Fermi (1901 - 1954).
cross-linking → umrežavanje
Cross-linking is an attachment of two chains of polymer molecules by bridges, composed of either an element, a group, or a compound, that join certain carbon atoms of the chains by primary chemical bonds, as indicated in the schematic diagram
Cross-linking occurs in nature in substances made up of polypeptide chains that are joined by the disulfide bonds of the cysteine residue, as in keratins or insulin. Cross-linking can be artificially effected, either adding a chemical substance (cross-linking agent), or by subjecting the polymer to high-energy radiation. Examples are: vulcanisation of rubber with sulphur, cross-linking of polystyrene with divinylbenzene, or cross-linking of polyethylene by means of high-energy radiation.
Cross-linking has the effect of changing a plastic from thermoplastic to thermosetting. Thus, it also increases strength, heat and electrical resistance, and especially resistance to solvents and other chemicals.
cysteine → cistein
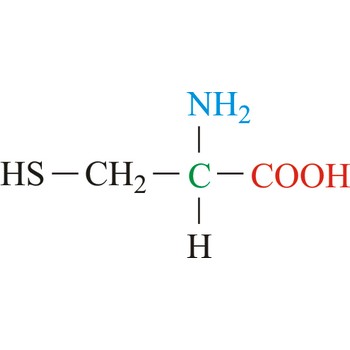
Cysteine is neutral amino acids with polar side chains. Because of its high reactivity, the thiol group of cysteine has numerous biological functions. It serves as a potent nucleophile and metal ligand (particularly for iron and zinc), but is best known for its ability to form disulfide bonds, which often make an important contribution to the stability of extracellular proteins. Cysteine is a non-essential amino acid, which means that it is biosynthesized in humans.
- Abbreviations: Cys, C
- IUPAC name: 2-amino-3-sulfanylpropanoic acid
- Molecular formula: C3H7NO2S
- Molecular weight: 121.16 g/mol
hydrophilic → hidrofilan
Hydrophilic is having a strong tendency to bind or absorb water, which results in swelling and formation of reversible gels. This property is characteristic of carbohydrates.
Citing this page:
Generalic, Eni. "Teorija valentne veze." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table