valence bond theory → teorija valentne veze
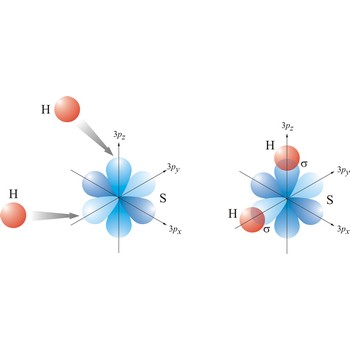
Valence bond theory is a theory that explains the shapes of molecules in terms of overlaps between half-filled atomic orbitals, or half filled hybridised orbitals.
valence bond → valentna veza
In the valence bond theory, a valence bond is a chemical bond formed by overlap of half-filled atomic orbitals on two different atoms.
collision theory → teorija sudara
Collision theory is theory that explains how chemical reactions take place and why rates of reaction alter. For a reaction to occur the reactant particles must collide. Only a certain fraction of the total collisions cause chemical change; these are called successful collisions. The successful collisions have sufficient energy (activation energy) at the moment of impact to break the existing bonds and form new bonds, resulting in the products of the reaction. Increasing the concentration of the reactants and raising the temperature bring about more collisions and therefore more successful collisions, increasing the rate of reaction.
Bronsted-Lowry’s acid-base theory → Bronsted-Lowryeva teorija kiselina i lužina
Brønsted-Lowry’s acid-base theory: Acid is a substance which gives a proton (protondonor) and base is a substance which accepts a proton (protonacceptor).
Dalton’s atomic theory → Daltonova atomska teorija
Dalton’s atomic theory is a theory of chemical combination, first stated by John Dalton in 1803. It involves the following postulates:
1. Elements consist of indivisible small particles (atoms).
2. All atoms of the same element are identical; different elements have different types of atom.
3. Atoms can neither be created nor destroyed.
4. ’Compound elements’ (i.e. compounds) are formed when atoms of different elements join in simple ratios to form ’compound atoms’ (i.e. molecules).
Dalton also proposed symbols for atoms of different elements (later replaced by the present notation using letters).
kinetic theory → kinetička teorija
Kinetic theory explains the behaviour of solids, liquids and gases and their state changes dependable upon motion of particles they are made of.
ligand field theory → teorija ligandnog polja
Ligand field theory is a description of the structure of crystals containing a transition metal ion surrounded by nonmetallic ions (ligands). It is based on the construction of molecular orbitals involving the d-orbitals of the central metal ion and combinations of atomic orbitals of the ligands.
sigma bond → sigma veza
Most single bonds are sigma bonds (σ-bond). In the valence bond theory, a sigma bond is a valence bond that is symmetrical around the imaginary line between the bonded atoms.
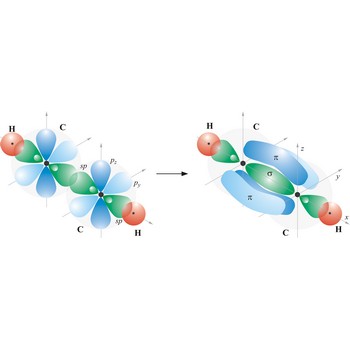
triple bond → trostruka veza
Triple bond. (≡) is a covalent bond that involves 3 bonding pairs. In the valence bond theory, one of the bonds in a triple bond is a sigma bond and the other two are pi bonds. For example, the central bond in acetylene is a triple bond: H-C≡C-H.
Citing this page:
Generalic, Eni. "Teorija valentne veze." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table