ultraviolet light → ultraljubičasto svjetlo
Ultraviolet light (UV light or UV radiation) is an electromagnetic radiation with a wavelength longer than that of x-rays, but shorter than that of visible light. Ultraviolet light can break some chemical bonds and cause cell damage.
light year → svjetlosna godina
Light year (ly) is a unit of distance used in astronomy, defined as the distance light travels in one year in a vacuum (ly = 9.46052973·1015 m).
luminous flux → svjetlosni tok
Luminous flux (Φ) is the intensity of light from a source multiplied by the solid angle. The SI unit is lumen.
polarised light → polarizirana svjetlost
Polarised light is light whose electromagnetic waves oscillate only in one flat vertical on expansion of the direction of radiation.
white light → bijela svjetlost
White light is a mixture of lights of all colours. If white light is passed through a glass prism or an optical lattice, it is separated into several colours (the visible light spectrum).
absorbance → apsorbancija
Absorbance (A) is a logarithm of the ratio of incident radiant power (Po) to transmitted radiant power (P) through a sample (excluding the effects on cell walls).
The absorption of light by a substance in a solution can be described mathematically by the Beer-Lambert law
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
Beer’s law → Beerov zakon
Beer’s law (or Beer-Lambert law) is the functional relationship between the quantity measured in an absorption method (A) and the quantity sought, the analyte concentration (c). As a consequence of interactions between the photons and absorbing particles, the power of the beam is attenuated from Po to P. Beer’s law can be written
where A is the absorbance at a given wavelength of light, ε is the molar absorbtivity or extinction coefficient (L mol-1 cm-1), unique to each molecule and varying with wavelength, b is the length of light path through the sample (cm), and c is the concentration of the compound in solution (mol L-1).
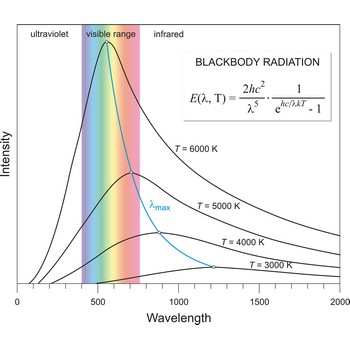
blackbody radiation → zračenje crnog tijela
Blackbody radiation is the radiation emitted by a perfect blackbody, i.e., a body which absorbs all radiation incident on it and reflects none. The primary law governing blackbody radiation is the Planck Radiation Law, which governs the intensity of radiation emitted by unit surface area into a fixed direction (solid angle) from the blackbody as a function of wavelength for a fixed temperature. The Planck Law can be expressed through the following equation
where λ is the wavelength, h is Planck’s constant, c is the speed of light, k is the Boltzmann constant, and T is the temperature.
Citing this page:
Generalic, Eni. "Svjetlo." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table