standard mean ocean water → standardna prosječna oceanska voda
Standard mean ocean water (SMOW) is a standard sample of pure water of accurately known isotopic composition which is maintained by the International Atomic Energy Agency. It is used for precise calibration of density and isotopic composition measurements.
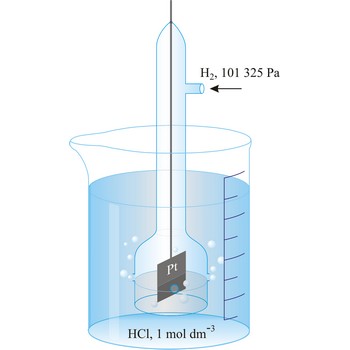
standard hydrogen electrode → standardna vodikova elektroda
Standard hydrogen electrode is a system in which hydrogen ion and gaseous hydrogen are present in their standard states. The convention is to designate the cell so that the standard hydrogen electrode is written first.
The electrode is used as a reference (of zero) for the values of other standard electrode potentials.
composition of ocean water → sastav oceanske vode
The proportions of the major constituents of ocean water are almost constant throughout the world. Salinity (total salt content) and the concentrations of individual chemical constituents in sea wateris given the units psu (practical salinity units). For most purposes one can assume that the new unit, psu, and the older unit, ‰, are synonymous.
The average composition of the ocean water is as shown on the following table.
| Constituent | Percentage of total salt |
|---|---|
| Chlorine | 55.3 % |
| Sodium | 30.8 % |
| Magnesium | 3.7 % |
| Sulphur | 2.6 % |
| Calcium | 1.2 % |
| Potassium | 1.1 % |
deionised water → deionizirana voda
Deionised water is water from which ionic salts have been removed by ion-exchange. It is used for many purposes as an alternative to distilled water.
| Type of water | Conductivity / µScm-1 |
|---|---|
| Ultrapure water | 0.05 |
| Distilled water | 0.5 |
| Tap water | 50 |
| Ocean water | 50 000 |
standard deviation → standardna devijacija
Standard deviation (σ) is a measure of the dispersion of a set of data from its mean. Standard deviation is a statistical term that measures the amount of variability or dispersion around an average
Suppose there are many measurements of a quantity presumed to be similar, like the size of peas in a pod. If the number of readings for each size were plotted, a bell-shaped curve would probably result, with a few small and large peas and most clustered around the average size. Around two-thirds of all measurements fall in the range spanned by the standard deviation, a measure of the spread.
average rate of reaction → prosječna brzina reakcije
Average rate of reaction is calculated in a way that a total change of reactants and products concentration is divided with time which is needed for reaction to end.
crystal water → kristalna voda
Crystal water is water contained in certain salt crystals. It can be removed by heating. Crystals that contain crystal water are called hydrated and their salts hydrates.
mineral water → mineralna voda
Mineral water is a groundwater that rises to the surface through a natural opening in the earth or rock and contains a relatively high concentration of mineral ions and trace of elements which can be radioactive or thermal.
distilled water → destilirana voda
Distilled water is water purified by distillation so as to free it from dissolved salts and other compounds. Distilled water in equilibrium with the carbon dioxide in the air has conductivity of about 0.8×10-6 S cm-1. Repeated distillation in vacuum can bring conductivity down to 0.043×10-6 S cm-1 at 18 °C. The limiting conductivity is due to self ionisation
soft water → meka voda
Soft water is any water that does not contain large concentrations of the dissolved minerals calcium or magnesium.
Citing this page:
Generalic, Eni. "Standardna prosječna oceanska voda." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table