universal gas constant → univerzalna plinska konstanta
Universal gas constant R has the value of 8.314 472(15) J K-1 mol-1. It corresponds to the volume work performed by one mole of gas heated by 1 K at standard pressure.
chlorinity → klorinitet
Originally chlorinity (symbol Cl) was defined as the weight of chlorine in grams per kilogram of seawater after the bromides and iodides had been replaced by chlorides. To make the definition independent of atomic weights, chlorinity is now defined as 0.3285233 times the weight of silver equivalent to all the halides.
The Mohr-Knudsen titration method served oceanographers for more than 60 years to determine salinity from chlorinity. This modification of the Mohr method uses special volumetric glassware calibrated directly in chlorinity units. The Mohr method uses potassium chromate (K2CrO4) as an indicator in the titration of chloride ions chloride (plus a small amount of bromide and iodide) with a silver nitrate (AgNO3) standard solution.
The other halides present are similarly precipitated.
A problem in the Mohr titration was that silver nitrate is not well suited for a primary standard. The Danish physicist Martin Knudsen (1871-1949) suggested that a standard seawater (Eau de mer Normale or Copenhagen Normal Water) be created and distributed to oceanographic laboratories throughout the world. This water was then used to standardize the silver nitrate solutions. In this way all chlorinity determinations were referred to one and the same standard which gave great internal consistency.
The relationship between chlorinity Cl and salinity S as set forth in Knudsen's tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
dielectric constant → dielektrična konstanta
Dielectric constant or permittivity (ε) is an index of the ability of a substance to attenuate the transmission of an electrostatic force from one charged body to another. The lower the value, the greater the attenuation. The standard measurement apparatus utilises a vacuum whose dielectric constant is 1. In reference to this, various materials interposed between the charged terminal have the following value at 20 °C:
| vacuum | 1 |
| air | 1.00058 |
| glass | 3 |
| benzene | 2.3 |
| acetic acid | 6.2 |
| ammonia | 15.5 |
| ethanol | 25 |
| glycerol | 56 |
| water | 81 |
The exceptionally high value for water accounts for its unique behaviour as a solvent and in electrolytic solutions. Dielectric constant values decrease as the temperature rises.
electrochemical series → elektrokemijski niz
Electrochemical series is a series of chemical elements arranged in order of their standard electrode potentials. The hydrogen electrode
is taken as having zero electrode potential. An electrode potential is, by definition, a reduction potential.
Elements that have a greater tendency than hydrogen to lose electrons to their solution are taken as electropositive; those that gain electrons from their solution are below hydrogen in the series and are called electronegative.
The series shows the order in which metals replace one another from their salts; electropositive metals will replace hydrogen from acids.
electrode potential → elektrodni potencijal
Electrode potential is defined as the potential of a cell consisting of the electrode in question acting as a cathode and the standard hydrogen electrode acting as an anode. Reduction always takes place at the cathode, and oxidation at the anode. According to the IUPAC convention, the term electrode potential is reserved exclusively to describe half-reactions written as reductions. The sign of the half-cell in question determines the sign of an electrode potential when it is coupled to a standard hydrogen electrode.
Electrode potential is defined by measuring the potential relative to a standard hydrogen half cell
The convention is to designate the cell so that the oxidised form is written first. For example
The e.m.f. of this cell is
By convention, at p(H2) = 101325 Pa and a(H+) = 1.00, the potential of the standard hydrogen electrode is 0.000 V at all temperatures. As a consequence of this definition, any potential developed in a galvanic cell consisting of a standard hydrogen electrode and some other electrode is attributed entirely to the other electrode
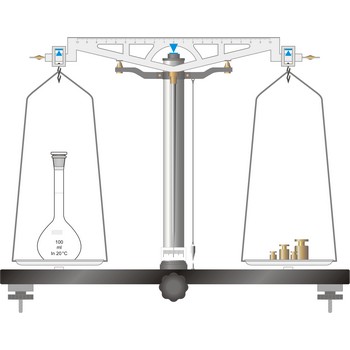
equal-arm balance → vaga s jednakim krakovima
The simplest type of balance, the equal-arm balance, is an application of a first class lever. The beam of the balance is supported on a central knife-edge, usually of agate, which rests upon a plane agate plate. The point of support is called the fulcrum. Two pans of equal weight are suspended from the beam, one at each end, at points equidistant from the fulcrum. A long pointer attached at right angles to the beam at the fulcrum indicates zero on a scale when the beam is at rest parallel to a level surface.
To prevent the knife-edge from becoming dull under the weight of the beam and pans the balance is equipped with a special device called an arrest. The arrest is operated by means of milled knob underneath the base plate in the middle and in front of the balance (sometimes the arrest knob is at one side of the balance).
The object to be weighed is placed on one pan, and standard weights are added to the other until the balance of the beam is established again. When not in use and during loading or unloading of the pans, the balance should be arrested.
fugacity → fugacitet
Fugacity (f) is a thermodynamic function used in place of partial pressure in reactions involving real gases and mixtures. For a component of a mixture, it is defined by
where μ is the chemical potential.
The fugacity of a gas is equal to the pressure if the gas is ideal. The fugacity of a liquid or solid is the fugacity of the vapour with which it is in equilibrium. The ratio of the fugacity to the fugacity in some standard state is the activity.
gravimetry → gravimetrija
Gravimetry is the quantitative measurement of an analyte by weighing a pure, solid form of the analyte. Since gravimetric analysis is an absolute measurement, it is the principal method for analysing and preparing primary standards.
A typical experimental procedure to determine an unknown concentration of an analyte in a solution is as follows:
- quantitatively precipitate the analyte from the solution
- collect the precipitate by filtering and wash it to remove impurities
- dry the solid in an oven to remove the solvent
- weigh the solid on an analytical balance
- calculate the analyte concentration in the original solution based on the weight of the precipitate.
equilibrium constant → konstanta ravnoteže
The equilibrium constant (K) was originally introduced in 1863 by Norwegian chemists C.M. Guldberg and P. Waage using the law of mass action. For a reversible chemical reaction represented by the equation
chemical equilibrium occurs when the rate of the forward reaction equals the rate of the back reaction, so that the concentrations of products and reactants reach steady-state values.
The equilibrium constant is the ratio of chemical activities of the species A, B, C, and D at equilibrium.
To a certain approximation, the activities can be replaced by concentrations.
For gas reactions, partial pressures are used rather than concentrations
The units of Kp and Kc depend on the numbers of molecules appearing in the stoichiometric equation (a, b, c, and d).
The value equilibrium constant depends on the temperature. If the forward reaction is exothermic, the equilibrium constant decreases as the temperature rises. The equilibrium constant shows the position of equilibrium. A low value of K indicates that [C] and [D] are small compared to [A] and [B]; i.e. that the back reaction predominates.
The equilibrium constant is related to ΔrG°, the standard Gibbs free energy change in the reaction, by
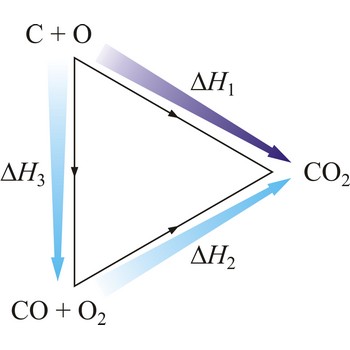
Hesse’s law → Hessov zakon
Hesse’s law says that reaction heat of some chemical change does not depend on the way in which the reaction is conducted, but only on starting and ending system state. Hesse’s law is also known as the law of constant heat summation. Hesse’s law is also known as the law of constant heat summation. The law was first put forward in 1840 by the Swiss-born Russian chemist Germain Henri Hess (1802-1850).
Hesse’s law can be used to obtain thermodynamic data that cannot be measured directly. For example, it is very difficult to control the oxidation of graphite to give pure CO. However, enthalpy for the oxidation of graphite to CO2 can easily be measured. So can the enthalpy of oxidation of CO to CO2. The application of Hess’s law enables us to estimate the enthalpy of formation of CO.
| C(s) + O2(g) →← CO2(g) | ΔrH1 = -393 kJ mol-1 |
| CO(g) + 1/2O2(g) →← CO2(g) | ΔrH2 = -283 kJ mol-1 |
| C(s) + 1/2O2(g) →← CO(g) | ΔrH3 = -110 kJ mol-1 |
The equation shows the standard enthalpy of formation of CO to be -110 kJ/mol.
Citing this page:
Generalic, Eni. "Standard." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table