dropping mercury electrode → kapajuća živina elektroda
Dropping mercury electrode (DME) is a working electrode arrangement for polarography in which mercury continuously drops from a reservoir through a capillary tube (internal diameter 0.03 - 0.05 mm) into the solution. The optimum interval between drops for most analyses is between 2 and 5 s. The unique advantage to the use of the DME is that the constant renewal of the electrode surface, exposed to the test solution, eliminates the effects of electrode poisoning.
heat capacity → toplinski kapacitet
Heat capacity is defined in general as dQ/dT, where dQ is the amount of heat that must be added to a system to increase its temperature by a small amount dT. The heat capacity at a constant pressure is Cp = (∂H/∂T)p; that at a constant volume is CV = (∂E/∂T)V, where H is enthalpy, E is internal energy, p is pressure, V is volume, and T is temperature. An upper case C normally indicates the molar heat capacity, while a lower case c is used for the specific (per unit mass) heat capacity.
iridium → iridij
Iridium was discovered by Smithson Tennant (England) in 1803. The origin of the name comes from the Latin word iris, meaning rainbow, because its salts are highly colored. It is heavy, brittle, white metal. Unreactive in air, water and acids. Attacked by fused NaOH. Metal ignites and burns readily. Iridium is found in gravel deposits with platinum. Used with osmium to tip gold pen points, to make crucible and special containers. Also to make alloys used for standard weights and measures and heat-resistant alloys. Also as hardening agent for platinum.
Knudsen’s pipette → Knudsenova pipeta
Kudsen's automatic pipette, developed by the Danish physicist Martin Knudsen (1871-1949), allows quick and accurate transfer of a constant volume of liquid (sea water), usually around 15 mL. On the top of pipette is a double sided C vent that can establish flow between the body of the pipette and one of the branches (A or B), or isolate the body of the pipette from both of the branches. Sucking through the B branch the pipette is filled with liquid, it is closed with a twist of the C valve and the liquid is released by rotating the valve towards the A branch (so atmospheric air can enter the pipette). Emptying the pipette takes around 30 seconds. Before it's first use, the pipette must be calibrated with distilled water.
Hesse’s law → Hessov zakon
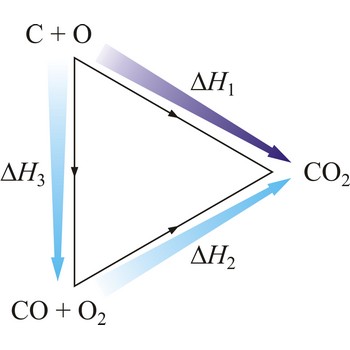
Hesse’s law says that reaction heat of some chemical change does not depend on the way in which the reaction is conducted, but only on starting and ending system state. Hesse’s law is also known as the law of constant heat summation. Hesse’s law is also known as the law of constant heat summation. The law was first put forward in 1840 by the Swiss-born Russian chemist Germain Henri Hess (1802-1850).
Hesse’s law can be used to obtain thermodynamic data that cannot be measured directly. For example, it is very difficult to control the oxidation of graphite to give pure CO. However, enthalpy for the oxidation of graphite to CO2 can easily be measured. So can the enthalpy of oxidation of CO to CO2. The application of Hess’s law enables us to estimate the enthalpy of formation of CO.
| C(s) + O2(g) →← CO2(g) | ΔrH1 = -393 kJ mol-1 |
| CO(g) + 1/2O2(g) →← CO2(g) | ΔrH2 = -283 kJ mol-1 |
| C(s) + 1/2O2(g) →← CO(g) | ΔrH3 = -110 kJ mol-1 |
The equation shows the standard enthalpy of formation of CO to be -110 kJ/mol.
lactic acid → mliječna kiselina
Lactic acid is an acid produced as a result of anaerobic respiration in muscles and red blood cells, i.e. when glycogen is used as an energy source for respiration rather than oxygen. After production, it is converted back to glycogen in the liver. The build up of large amounts of lactic acid in the blood can lead to stress and toxic effects. High levels are usually a result of sustained, excessive exercise.
manganese → mangan
Manganese was discovered by Johann Gahn (Sweden) in 1774. The origin of the name comes from the Latin word magnes meaning magnet, or magnesia nigri meaning black magnesia (MnO2). It is hard, brittle, grey-white metal with a pinkish tinge. Impure forms are reactive. Rusts like iron in moist air. Manganese is most abundant ores are pyrolusite (MnO2), psilomelane [(Ba,H2O)2Mn5O10] and rhodochrosite (MnCO3). Pure metal produced by mixing MnO2 with powered Al and ignited in a furnace. Used in steel, batteries and ceramics. The steel in railroad tracks can contain as much as 1.2 % manganese. It is crucial to the effectiveness of vitamin B1.
mass → masa
Mass (m) is the quantity of matter contained in a particle or body regardless of its location in the universe. Mass is constant, whereas weight is affected by the distance of a body from the centre of the Earth (or of other planet). The SI unit is kilogram.
According to the Einstein equation
all forms of energy possess a mass equivalent.
Moh’s scale → Mohsova skala
Mohs’ scale of mineral hardness characterises the scratch resistance of various minerals through the ability of a harder material to scratch a softer. It was created by the German mineralogist Friedrich Mohs (1773-1839). Mohs based the scale on the ten readily available minerals.
| Hardness | Mineral |
|---|---|
| 1 | talc (Mg3Si4O10(OH)2) |
| 2 | gypsum (CaSO4·2H2O) |
| 3 | calcite (CaCO3) |
| 4 | fluorite (CaF2) |
| 5 | apatite (Ca5(PO4)3(OH-,Cl-,F-)) |
| 6 | orthoclase feldspar (KAlSi3O8) |
| 7 | quartz (SiO2) |
| 8 | topaz (Al2SiO4(OH-,F-)2) |
| 9 | corundum (Al2O2) |
| 10 | diamond (C) |
molybdenum → molibden
Molybdenum was discovered by Carl William Scheele (Sweden) in 1778. The origin of the name comes from the Greek word molybdos meaning lead. It is silvery white, very hard metal, but is softer and more ductile than tungsten. Molybdenum is found in the minerals molybdenite (MoS2) and wulfenite (MoO4Pb). Its alloys are used in aircraft, missiles and protective coatings in boiler plate.
Citing this page:
Generalic, Eni. "Stalna tvrdoća." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table