Allihn condenser → Allihnovo hladilo
Allihn condenser or bulb condenser consists of an outer water jacket and the inner glass tube with a series of spherical bubbles to maximize the thermal contact with the cooling water. It is named after its inventor, the German chemist Felix Richard Allihn (1854-1915).
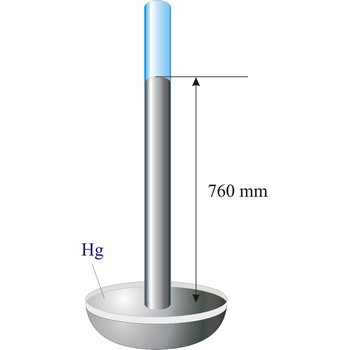
barometer → barometar
Barometer is an instrument that measures atmospheric pressure. A mercury barometer is a closed tube filled with mercury inverted in a mercury reservoir. The height of the mercury column indicates atmospheric pressure (with 1 atm = 760 mm of mercury). An aneroid barometer consists of an evacuated container with a flexible wall. When atmospheric pressure changes, the wall flexes and moves a pointer which indicates the changing pressure on a scale.
boron → bor
Boron compounds have been known for thousands of years, but the element was not discovered until 1808 by Sir Humphry Davy (England) and independently by Joseph-Louis Gay-Lussac (France) and L. J. Thenard (France). The origin of the name comes from the Arabic word buraq and the Persian word burah meaning boraks. It is hard, brittle, lustrous black semimetal. Unreactive with oxygen, water, alkalis or acids. Combines with most metals to form borides. Boron is obtained from kernite, a kind of borax (Na2B4O7·10H2O). High purity boron is produced by electrolysis of molten potassium fluroborate and potassium chloride (KCl). Amorphous boron is used in pyrotechnic flares to provide a distinctive green color and in rockets as an igniter.
analytical balance → analitička vaga
Analytical balances are instruments used for precise determining mass of matter. Analytical balances are sensitive and expensive instruments, and upon their accuracy and precision the accuracy of analysis result depends. The most widely used type of analytical balances are balances with a capacity of 100 g and a sensitivity of 0.1 mg. Not one quantitative chemical analysis is possible without usage of balances, because, regardless of which analytical method is being used, there is always a need for weighing a sample for analysis and the necessary quantity of reagents for solution preparation.
The working part of the balance is enclosed in a glass-fitted case. The baseplate is usually of black glass or black slate. The beam has agate knife-edges at its extremes, supporting stirrups from which balance pans are suspended. Another agate or steel knife-edge is fixed exactly in the middle of the beam on its bottom side. This knife-edge faces downwards and supports the beam. When not in use and during loading or unloading of the pans, the balance should be arrested.
The principle of operation of a modern laboratory balance bears some resemblance to its predecessor - the equal arm balance. The older instrument opposed the torque exerted by an unknown mass on one side of a pivot to that of an adjustable known weight on the other side. When the pointer returned to the center position, the torques must be equal, and the weight was determined by the position of the moving weights.
Modern electronic laboratory balances work on the principle of magnetic force restoration. In this system, the force exerted by the object being weighed is lifted by an electromagnet. A detector measures the current required to oppose the downward motion of the weight in the magnetic field.
Buchner funnel → Buchnerov lijevak
Büchner funnel is one device used for pressure assisted filtration. Buchner funnel is a cylindrical porcelain filtering funnel (glass and plastic funnels are also available) that has a perforated plate on which the flat filter paper is placed. A vacuum in the flask underneath the filter allows atmospheric pressure on the sample to force the liquid through the filter paper. It is named after the German chemist Ernst Wilhelm Büchner (1850-1925) who designed this funnel in 1885.
Bunsen, Robert Wilhem → Bunsen, Robert Wilhem
Robert Wilhem Bunsen (1811-1899) is a German chemist who held professorships at Kassel, Marburg and Heidelberg. His early researches on organometallic compound of arsenic cost him an eye in an explosion. Bunsen's most important work was in developing several techniques used in separating, identifying, and measuring various chemical substances. He also improvement chemical battery for use in isolating quantities of pure metals - Bunsen battery.
The essential piece of laboratory equipment that has immortalized the name of Bunsen was not invented by him. Bunsen improved the burner's design, which had been invented by Faraday, to aid his endeavors in spectroscopy. Use of the Bunsen burner in conjunction with a glass prism led to the development of the spectroscope in collaboration with the German physicist Gustav Kirchoff and to the spectroscopic discovery of the elements rubidium (1860) and cesium (1861).
burette → bireta
Burette is a graded glass pipe which on its lower side has a glass faucet by which it can drop a precise quantity of liquid. Inner diameter of a burette must be equal in its whole length, because the accuracy of volume measurement depends upon that. Burettes are primarily used in volumetric analysis for titration with standard solution reagent. Most often Schellbach’s burette is used, graded on 50 mL with division of scale on 0.1 mL. Every burette is calibrated on discharge. For serial determining automatic burettes are used.
cerium → cerij
Cerium was discovered by Martin Heinrich Klaproth (Germany) and by Jöns Jacob Berzelius (Sweden) in 1803 and Wilhelm von Hisinger (Germany) in 1814. Named after the asteroid Ceres this discovered two years before the element. It is malleable, ductile, iron-grey metal. Tarnishes in air; reacts easily with water. Dissolves in acids; ignites when heated. Metal ignites and burns readily. Strong reductant. Cerium is most abundant rare earth metal. Found in many minerals like monazite sand [Ce(PO4)]. Its oxides are used in the optics and glass-making industries. Its salts are used in the photography and textile industry. Used in high-intensity carbon lamps and as alloying agents in special metals.
Citing this page:
Generalic, Eni. "Staklo." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table