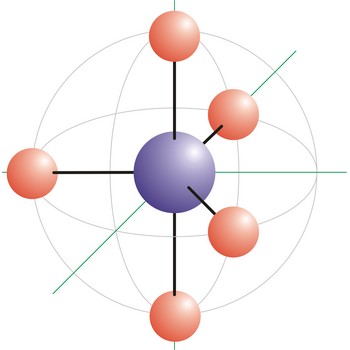
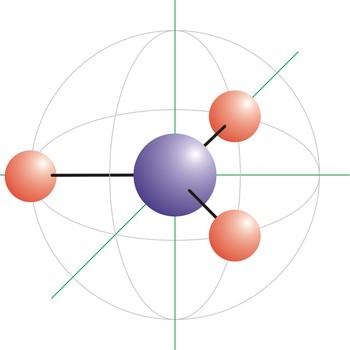
T-shaped molecular geometry → T-oblik geometrije molekule
T-shape is a molecular geometry that results when there are 3 bonds and 2 lone pairs around the central atom in the molecule. The atoms bonded to the central atom lie at the ends of a T with 90° angles between them. Molecules with an trigonal bipyramidal electron pair geometries have sp3d (or dsp3) hybridization at the central atom. ICl3 has a T-shaped molecular geometry.
tetrahedral molecular geometry → tetraedarska geometrija molekule
Tetrahedral is a molecular shape that results when there are four bonds and no lone pairs around the central atom in the molecule. The atoms bonded to the central atom lie at the corners of a tetrahedron with 109.5° angles between them. Molecules with an tetrahedral electron pair geometries have sp3 hybridization at the central atom. The ammonium ion (NH4+) and methane (CH4) have a tetrahedral molecular geometry.
trigonal bipyramidal molecular geometry → trigonska bipiramidalna geometrija molekule
Trigonal bipyramidal (trigonal bipyramidal shape) is a molecular geometry that results when there are five bonds and no lone pairs on the central atom in the molecule. Three of the bonds are arranged along the atom’s equator, with 120° angles between them; the other two are placed at the atom’s axis. Axial bonds are at right angles to the equatorial bonds. Molecules with an trigonal bipyramidal electron pair geometries have sp3d (or dsp3) hybridization at the central atom. The PCl5 molecule has a trigonal bipyramidal molecular geometry.
trigonal planar molecular geometry → trigonska planarna geometrija molekule
Trigonal planar is a molecular shape that results when there are three bonds and no lone pairs around the central atom in the molecule. The pairs are arranged along the central atom’s equator, with 120° angles between them. Molecules with an trigonal planar electron pair geometries have sp2d hybridization at the central atom. The carbonate ion (CO32-) has a trigonal planar geometry.
velocity → brzina
If a point-like object moves so that its position vector changes from being ri to rf, than the displacement Δr of object is
If a point-like object undergoes a displacement, Δr, in time Δt, its average velocity, v is defined as
The instantaneous velocity, v, is obtained from the average velocity by shrinking the time interval Δt towards zero. The average velocity approaches a limiting value, which is the velocity of a given instant:
Velocity is a vector quantity. If we plot the path of a moving particle as a curve in a coordinate system, the instantaneous velocity is always tangent to that curve.
SI unit for velocity is m s-1.
volumetric pipette → prijenosna pipeta
Volumetric pipettes (transfer or belly pipette) are used in volumetric analysis, when there is a need for taking exact smaller volume of a sample solution or reagent. The upper tube of volumetric pipette has a ringlike marking (mark) which marks its calibrated volume. Pipettes calibrated to deliver (TD or Ex) the indicated volume. By sucking in (with mouth, propipette or a water pump) the liquid is pulled in a little bit above the mark and the opening of the pipet is closed with a forefingertip. Outer wall of pipet is wiped and, with a slight forefinger loosening, the liquid is released until it reaches the mark. Mark must figure as a tangent on a lower edge of the liquid meniscus. A pipette is emptied out by lifting the forefinger off and letting the liquid flow out of the pipette freely. After another 15 s and the tip of the pipette is pulled onto the inner wall of the vessel. It is absolutely forbidden to blow out the contents of the pipette.
X-rays → X-zrake
X-rays are electromagnetic radiation of shorter wavelength than ultraviolet radiation (10-11 m to 10-9 m or 0.01 nm to 1 nm) produced by bombardment of atoms by high-quantum-energy particles. X-rays can pass through many forms of matter and they are therefore used medically and industrially to examine the internal structure.

Chitosan → Kitozan
Chitosan is a linear polysaccharide composed of randomly distributed N-acetyl D-glucosamine and D-glucosamine units. It can be easily derived from partial deacetylation of natural polymer chitin. At a minimum deacetylization level of 60 % (amount of free amino groups in the polymer) it is considered to be chitosan. Thanks to the amino groups of D-glucosamine, chitosan can be protonated and turned into polycation, which is one of the sources of unique properties of chitosan as biopolymer, like aqueous solubility, antibacterial properties, biodegradability with non-toxic residues and biocompatibility.
water hardness → tvrdoća vode
Hardness is defined as the concentrations of calcium and magnesium ions expressed in terms of calcium carbonate. These minerals in water can cause some everyday problems. They react with soap and produce a deposit called soap curd that remains on the skin and clothes and, because it is insoluble and sticky, cannot be removed by rinsing.
Hard water may also shorten the life of plumbing and water heaters. When water containing calcium carbonate is heated, a hard scale is formed that can plug pipes and coat heating elements. Scale is also a poor heat conductor. With increased deposits on the unit, heat is not transmitted to the water fast enough and overheating of the metal causes failure. Build-up of deposits will also reduce the efficiency of the heating unit, increasing the cost of fuel.
There are two types of water hardness, temporary and permanent.
Temporary Hardness is due to the bicarbonate ion, HCO3-, being present in the water. This type of hardness can be removed by boiling the water to expel the CO2, as indicated by the following equation:
Permanent hardness is due to calcium and magnesium nitrates, sulphates, and chlorides etc. This type of hardness cannot be eliminated by boiling.
| Water supply classification | |
|---|---|
| Hardness | Concentration of Calcium carbonate (mg/L) |
| Soft Water | 0 to 75 |
| Medium Hard Water | 75 to 150 |
| Hard Water | 150 to 300 |
| Very Hard Water | over 300 |
Citing this page:
Generalic, Eni. "Srednji slobodni put." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table