colloid silver → koloidno srebro
Colloid silver is a bright blue-green powder which dissolved in water gives colloid solution of red colour.
silver → srebro
Silver has been known since ancient times. The origin of the name comes from the Latin word argentum meaning silver. It is silvery-ductile and malleable metal. Stable in water and oxygen. Reacts with sulfur compounds to form black sulfides. Silver is found in ores called argentite (AgS), light ruby silver (Ag3AsS3), dark ruby silver (Ag3SbS3) and brittle silver. Used in alloys for jewellery and in other compounds for photography. It is also a good conductor, but expensive.
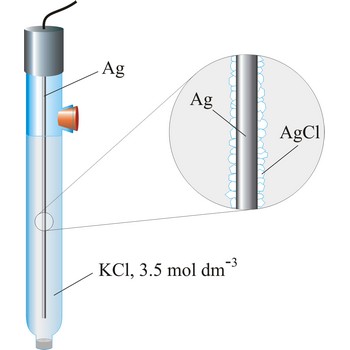
silver/silver-chloride electrode → srebro/srebrov klorid elektroda
Silver/silver-chloride electrode is by far the most common reference type used today because it is simple, inexpensive, very stable and non-toxic. It is mainly used with saturated potassium chloride electrolyte, but can be used with lower concentrations such as 3.5 mol dm-3 or 1 mol dm-3 potassium chloride. Silver/silver-chloride electrode is a referent electrode based on the following halfreaction
| Potential vs. SHE / V | ||
|---|---|---|
| t / °C | 3.5 mol dm-3 | sat. solution |
| 15 | 0.212 | 0.209 |
| 20 | 0.208 | 0.204 |
| 25 | 0.205 | 0.199 |
| 30 | 0.201 | 0.194 |
| 35 | 0.197 | 0.189 |
silver coulometer → srebrni kulometar
Silver coulometer consists of a platinum vessel which acts as a cathode and contains a solution of pure silver nitrate as an electrolyte (c(AgNO3) = 1 mol/L). A rod of pure silver enclosed in a porous pot acts as the anode. The current density at the anode should not exceed 0.2 Acm-2. After electrolysis, the electrolyte is taken out and the platinum vessel is washed, dried and weighed. The increase in the weight gives the amount of silver deposited (96500 C of electricity deposits 107.88 g of silver). From the mass of the silver deposited, the coulomb involved in the reaction can be calculated.
actinium → aktinij
Actinium was discovered by André Debierne (France) in 1899. The origin of the name comes from the Greek word aktinos meaning ray. It is heavy, silvery-white, very radioactive metal. Reacts with water. Glows in the dark. Actinium is extremely rare, found in all uranium ores. Usually obtained by treating radium with neutrons in a reactor.
alpaca → alpaka
Alpaca (alpaka) or Nickel Silver is the generic name for any of a range of non-precious bright silvery-grey metal alloys, composed of copper, nickel and zinc. Despite its name it contains no real silver. It is also commonly called German Silver.
There are many different formulations of alloys which fall within the general term of Nickel Silver. All contain copper, nickel and zinc, while some formulations may additionally include antimony, tin, lead or cadmium. A representative formulation is 65 % copper, 18 % nickel, 17 % zinc. Nickel Silver is widely used for the commercial production of industrial components, housewares, flatware and cutlery, and as the metal substrate for silver-plated goods.
aluminium → aluminij
Aluminium was discovered by Friedrich Wöhler (Germany) in 1827. The origin of the name comes from the Latin word alumen meaning alum. It is soft, lightweight, silvery-white metal. Exposed surfaces quickly form protective oxide coating. Metal reacts violently with oxidants. Third most abundant element in the earth’s crust. Aluminium is the most abundant metal to be found in the earth’s crust, but is never found free in nature. Aluminium is obtained by electrolysis from bauxite. Used for many purposes from airplanes to beverage cans. Too soft in its pure form so less than 1 % of silicon or iron is added, which hardens and strengthens it.
americium → americij
Americium was discovered by Glenn T. Seaborg, Ralph A. James, Stanley G. Thompson and Albert Ghiorso (USA) in 1944. Named for the American continent. It is silvery-white, artificially produced radioactive metal. Americium was produced by bombarding plutonium with neutrons. Americium-241 is currently used in smoke detectors.
arsenic → arsen
Arsenic was discovered by Albertus Magnus (Germany) in 1250. The origin of the name comes from the Greek word arsenikon meaning yellow orpiment. It is steel-grey, brittle semi-metal. Resists water, acids and alkalis. Tarnishes in air, burns in oxygen. Highly toxic by inhalation or ingestion. Arsenic is found in mispickel (arsenopyrite). Many of its compounds are deadly poison and used as weed killer and rat poison. Used in semiconductors. Some compounds, called arsenides, are used in the manufacture of paints, wallpapers and ceramics.
berkelium → berkelij
Berkelium was discovered by Stanley G. Thompson, Albert Ghiorso and Glenn T. Seaborg (USA) in 1949. Named after Berkeley, a city in California, home of the University of California, USA. It is synthetic radioactive metal. Berkelium was made by bombarding americium with alpha particles.
Citing this page:
Generalic, Eni. "Srebro." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table