filtration → filtriranje
Filtration is a procedure in which liquids are separated from the precipitate by passing a suspension through the filter. The precipitate remains on the filter and through it the filtrate passes. Gaseous heterogeneous mixtures can also be filtrated.
standardisation → standardizacija
Standardisation is a process of determining the exact concentration of secondary standard solution.
stereospecific reaction → stereospecifična reakcija
Stereospecific reactions are reactions that proceed predominantly to a single stereoisomeric product out. All metabolic conversions involving chiral molecules are stereospecific.
thermit welding → termitno zavarivanje
Thermit welding is a group of welding processes in which fusion is produced by heating with superheated liquid metal resulting from a chemical reaction between a metal oxide and aluminium.
toxic chemical → bojni otrov
Toxic chemical means any chemical which through its chemical action on life processes can cause death, temporary incapacitation, or permanent harm to humans or animals. This includes all such chemicals, regardless of their origin or of their method of production, and regardless of whether they are produced in facilities, in munitions or elsewhere.
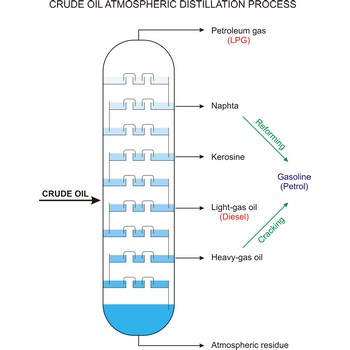
fractional distillation → frakcijska destilacija
Fractional distillation is a procedure in which liquids of close boiling points are separated. It is conducted in fraction or rectification columns in a way that vapour phase created by distillation is condensed and the condensate thus obtained is redistilled. The procedure is repeated several times. Vapour phase always contains more volatile component than the liquid phase, at top of the column vapour of clean volatile component gets out and at the bottom of the column liquid of nonvolatile component.
fuel cell → gorivi članak
Fuel cell is a device that converts chemical energy into electrical energy. It is different from a battery in that the energy conversion continues as long as fuel and oxidising agent are fed to the fuel cell; that is, in principle indefinitely. (A battery is manufactured with a limited amount of chemicals, and it is exhausted when all the chemicals have reacted.) It is a galvanic cell where spontaneous chemical reactions occur at the electrodes. The fuel is oxidised at the anode, and the oxidising agent (almost always oxygen or air) is reduced at the cathode. Presently, the most commonly used fuel is hydrogen. More conventional fuels (e.g., petrol or natural gas) must be converted (reformed) into hydrogen before they can be utilised in a fuel cell.
Some fuel cells employ an aqueous solution as electrolyte, that can be either acidic or basic (alkaline), or an ion-exchange membrane soaked in aqueous solution can act as the electrolyte. These fuel cells operate at relatively low temperatures (from room temperature to not much above the boiling point of water). Some fuel cells employ molten salts (especially carbonates) as electrolytes and have to operate at temperatures of several hundred degrees centigrade (Celsius). Others employ ionically conductive solids as electrolyte and must operate close to 1 000 °C.
transumutation → transmutacija
Transmutation is the conversion of one element into another by a process taking place in the nucleus.
vulcanisation → vulkanizacija
Vulcanisation (vulcanisation of rubber) is a process of combining rubber with sulphur or other substances that causes the polymer chains to crosslink, making them stronger and more elastic.
water purification → pročišćavanje vode
Water purification is a procedure of harmful substances removal in order to obtain pure drinking-water.
Citing this page:
Generalic, Eni. "Spontani proces." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table