Results 1–8 of 8 for slijepa proba
Biot-Savart law → Biot-Savartov zakon
The magnetic field B due to a current-carrying conductor can be determined by Biot-Savart law. The contribution to magnetic field set up at distance r by the current element IdL is given by expression:
where μ0 is permeability constant. It plays a role in magnetic problems equivalent to the role of permittivity constant μ0 in electrostatics problems. In order to obtain B, contributions of all current elements have to be integrated. In case of a long straight conductor, carrying current I, Biot-Savart law gives:
SI unit for magnetic field B is tesla (T).
Permaeability constant μ0 has value 4π×10-7 T m A-1.
Boltzmann equation → Boltzmannova jednadžba
Boltzmann equation is a statistical definition of entropy, given by
where S and k are the entropy and Boltzmann’s constant, respectively, and W is the probability of finding the system in a particular state.
cadmium → kadmij
Cadmium was discovered by Friedrich Strohmeyer (Germany) in 1817. The origin of the name comes from the Latin word cadmia meaning calamine (zinc carbonate, ZnCO3), or from the Greek word kadmeia with the same meaning. It is soft, malleable, blue-white metal. Tarnishes in air, soluble in acids, insoluble in alkalis. Boiling cadmium gives off a weird, yellow-colored vapour that is poisonous. Cadmium can cause a variety of health problems, including kidney failure and high blood pressure. Cadmium is obtained as a by product of zinc refining. The mayor use of cadmium is in electroplating of steel to protect it from corrosion. Also used to make nickel-cadmium batteries. The ability of cadmium to adsorb neutrons has made it of great importance in the design of nuclear reactors. Its compounds are found in paint pigments and a wide variety of intense colours.
quantum chemistry → kvantna kemija
Quantum chemistry is a theoretical branch of chemistry that concerns the application of quantum mechanics to chemical problems.
chlorinity → klorinitet
Originally chlorinity (symbol Cl) was defined as the weight of chlorine in grams per kilogram of seawater after the bromides and iodides had been replaced by chlorides. To make the definition independent of atomic weights, chlorinity is now defined as 0.3285233 times the weight of silver equivalent to all the halides.
The Mohr-Knudsen titration method served oceanographers for more than 60 years to determine salinity from chlorinity. This modification of the Mohr method uses special volumetric glassware calibrated directly in chlorinity units. The Mohr method uses potassium chromate (K2CrO4) as an indicator in the titration of chloride ions chloride (plus a small amount of bromide and iodide) with a silver nitrate (AgNO3) standard solution.
The other halides present are similarly precipitated.
A problem in the Mohr titration was that silver nitrate is not well suited for a primary standard. The Danish physicist Martin Knudsen (1871-1949) suggested that a standard seawater (Eau de mer Normale or Copenhagen Normal Water) be created and distributed to oceanographic laboratories throughout the world. This water was then used to standardize the silver nitrate solutions. In this way all chlorinity determinations were referred to one and the same standard which gave great internal consistency.
The relationship between chlorinity Cl and salinity S as set forth in Knudsen's tables is
In 1962, however, a better expression for the relationship between total dissolved salts and chlorinity was found to be
orbital → orbitala
Orbital is the area in space about an atom or molecule in which the probability of finding an electron is greatest.
The possible atomic orbitals correspond to subshells of the atom. Thus there is one s-orbital for each shell (orbital quantum number l = 0). There are three p-orbitals (corresponding to the three values of l) and five d-orbitals. The shapes of orbitals depend on the value of l.
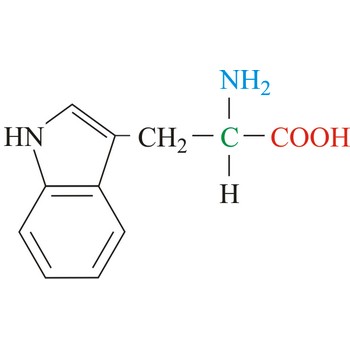
tryptophan → triptofan
Tryptophan is hydrophobic amino acids with aromatic side chain. Tryptophan is large aromatic residue that is normally found buried in the interior of a protein and is important for protein stability. Tryptophan has the largest side chain and is the least common amino acid in proteins. It has spectral properties that make it the best inherent probe for following protein folding and conformational changes associated with biochemical processes. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested.
- Abbreviations: Trp, W
- IUPAC name: 2-amino-3-(1H-indol-3-yl)propanoic acid
- Molecular formula: C11H12N2O2
- Molecular weight: 204.23 g/mol
water hardness → tvrdoća vode
Hardness is defined as the concentrations of calcium and magnesium ions expressed in terms of calcium carbonate. These minerals in water can cause some everyday problems. They react with soap and produce a deposit called soap curd that remains on the skin and clothes and, because it is insoluble and sticky, cannot be removed by rinsing.
Hard water may also shorten the life of plumbing and water heaters. When water containing calcium carbonate is heated, a hard scale is formed that can plug pipes and coat heating elements. Scale is also a poor heat conductor. With increased deposits on the unit, heat is not transmitted to the water fast enough and overheating of the metal causes failure. Build-up of deposits will also reduce the efficiency of the heating unit, increasing the cost of fuel.
There are two types of water hardness, temporary and permanent.
Temporary Hardness is due to the bicarbonate ion, HCO3-, being present in the water. This type of hardness can be removed by boiling the water to expel the CO2, as indicated by the following equation:
Permanent hardness is due to calcium and magnesium nitrates, sulphates, and chlorides etc. This type of hardness cannot be eliminated by boiling.
| Water supply classification | |
|---|---|
| Hardness | Concentration of Calcium carbonate (mg/L) |
| Soft Water | 0 to 75 |
| Medium Hard Water | 75 to 150 |
| Hard Water | 150 to 300 |
| Very Hard Water | over 300 |
Citing this page:
Generalic, Eni. "Slijepa proba." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table