volumetric pipette → prijenosna pipeta
Volumetric pipettes (transfer or belly pipette) are used in volumetric analysis, when there is a need for taking exact smaller volume of a sample solution or reagent. The upper tube of volumetric pipette has a ringlike marking (mark) which marks its calibrated volume. Pipettes calibrated to deliver (TD or Ex) the indicated volume. By sucking in (with mouth, propipette or a water pump) the liquid is pulled in a little bit above the mark and the opening of the pipet is closed with a forefingertip. Outer wall of pipet is wiped and, with a slight forefinger loosening, the liquid is released until it reaches the mark. Mark must figure as a tangent on a lower edge of the liquid meniscus. A pipette is emptied out by lifting the forefinger off and letting the liquid flow out of the pipette freely. After another 15 s and the tip of the pipette is pulled onto the inner wall of the vessel. It is absolutely forbidden to blow out the contents of the pipette.
zirconium → cirkonij
Zirconium was discovered by Martin Heinrich Klaproth (Germany) in 1789. The origin of the name comes from the Arabic word zargun meaning gold colour. It is grey-white, lustrous, corrosion-resistant metal. Exposed surfaces form oxide protective film. Zirconium is found in many minerals such as zircon and baddeleyite. Used in alloys such as zircaloy this is used in nuclear applications since it does not readily absorb neutrons. Also baddeleyite is used in lab crucibles. Used in high-performance pumps and valves. Clear zircon (ZrSiO4) is a popular gemstone.
zwitterion → dipolarni ion
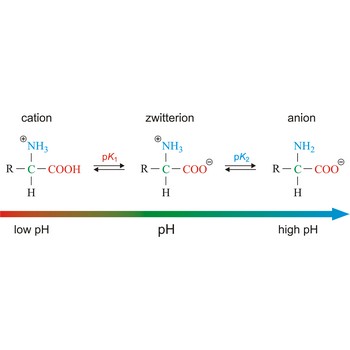
Zwitterion, also known as inner salt or dipolar ion, is an ion with a positive and a negative electrical charge at different locations within a molecule. As the molecule contains two opposite charges, it is electrically neutral. The term zwitterion is derived from the German word zwitter, meaning a hybrid, hermaphrodite. Zwitterions can be formed from compounds that contain both acid groups and base groups in their molecules (ampholytes).
All of the common amino acids found in proteins are ampholytes because they contain a carboxyl group (-COOH) that acts as an acid and an amino group (-NH2) that acts as a base. In the solid state, amino acids exist in the dipolar or zwitterion form. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (H+) ion, and the amino acid becomes positively charged. If base is added, ion removal of the H+ ion from the amino group of the zwitterion produces a negatively charged amino acid.
Chitin → Hitin
Chitin is a nitrogen-containing linear polysaccharide of ß(1->4) linked units of N-acetyl-ß-d-glucosamine. The structure of chitin is similar to cellulose except for the replacement hydroxyl group (-OH) at the carbon 2 with an acetyl amine group (–NH–CO–CH3). Chitin is the main component of the exoskeleton, or outer covering of insects, crustaceans, and arachnids. It is also found in the cell walls of certain fungi and algae. After cellulose, chitin is the second most abundant biopolymer in nature. It is insoluble in water, organic solvents, weak acids and lyes.
Cystine → Cistin
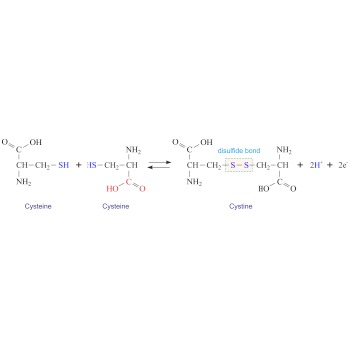
Cystine (C6H12N2O4S2) is a dimer of cysteine. It is formed by the oxidation of the thiol groups (-SH) of two cysteines generating a disulphide bridge (-S-S-). Cystine is a white crystalline solid that is slightly soluble in water. Cystine is particularly abundant in skeletal and connective tissues and in hair, horn, and wool.
Citing this page:
Generalic, Eni. "Slaba baza." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table