valine → valin
Valine is hydrophobic amino acids with aliphatic side chain. It is a member of the branched-chain amino acid family, along with leucine and isoleucine. Valine differs from threonine by replacement of the hydroxyl group with a methyl substituent, but they are of roughly the same shape and volume. The nonpolar hydrophobic amino acids tend to cluster together within proteins, stabilizing protein structure by means of hydrophobic interactions. Valine is an essential amino acid, which means that it cannot be synthesized in the body and must be obtained through dietary sources.
- Abbreviations: Val, V
- IUPAC name: 2-amino-3-methylbutanoic acid
- Molecular formula: C5H11NO2
- Molecular weight: 117.15 g/mol
velocity → brzina
If a point-like object moves so that its position vector changes from being ri to rf, than the displacement Δr of object is
If a point-like object undergoes a displacement, Δr, in time Δt, its average velocity, v is defined as
The instantaneous velocity, v, is obtained from the average velocity by shrinking the time interval Δt towards zero. The average velocity approaches a limiting value, which is the velocity of a given instant:
Velocity is a vector quantity. If we plot the path of a moving particle as a curve in a coordinate system, the instantaneous velocity is always tangent to that curve.
SI unit for velocity is m s-1.
yttrium → itrij
Yttrium was discovered by Carl Gustaf Mosander (Sweden) in 1843. Named after Ytterby, a village in Sweden. It is silvery, ductile, fairly reactive metal. Exposed surfaces form oxide film. Easily combustible, reacts with oxygen in water to release hydrogen. Yttrium is found in minerals such as monazite, xenotime and yttria. Combined with europium to make red phosphors for colour TV’s. Yttrium oxide and iron oxide combine to form a crystal garnet used in radar.
zwitterion → dipolarni ion
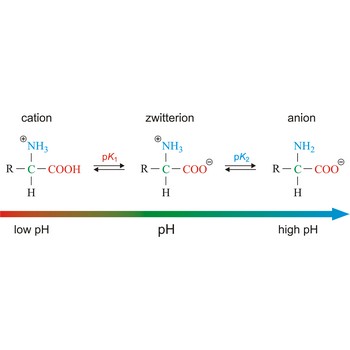
Zwitterion, also known as inner salt or dipolar ion, is an ion with a positive and a negative electrical charge at different locations within a molecule. As the molecule contains two opposite charges, it is electrically neutral. The term zwitterion is derived from the German word zwitter, meaning a hybrid, hermaphrodite. Zwitterions can be formed from compounds that contain both acid groups and base groups in their molecules (ampholytes).
All of the common amino acids found in proteins are ampholytes because they contain a carboxyl group (-COOH) that acts as an acid and an amino group (-NH2) that acts as a base. In the solid state, amino acids exist in the dipolar or zwitterion form. If acid is added to a solution containing the zwitterion, the carboxylate group captures a hydrogen (H+) ion, and the amino acid becomes positively charged. If base is added, ion removal of the H+ ion from the amino group of the zwitterion produces a negatively charged amino acid.
Chitosan → Kitozan
Chitosan is a linear polysaccharide composed of randomly distributed N-acetyl D-glucosamine and D-glucosamine units. It can be easily derived from partial deacetylation of natural polymer chitin. At a minimum deacetylization level of 60 % (amount of free amino groups in the polymer) it is considered to be chitosan. Thanks to the amino groups of D-glucosamine, chitosan can be protonated and turned into polycation, which is one of the sources of unique properties of chitosan as biopolymer, like aqueous solubility, antibacterial properties, biodegradability with non-toxic residues and biocompatibility.
Heyrovsky-Ilkovic equation → Heyrovsky-Ilkovičeva jednadžba
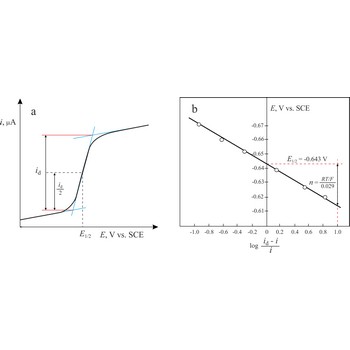
The Heyrovsky-Ilkovic equation describes the entire current-potential curve (polarographic wave) of a reversible redox system in polarography
where R is the gas constant, T is the absolute temperature, F is the Faraday constant, n denotes the number of electrons taking part in the electrode reaction. E1/2 is a unique potential (for a given reaction and supporting electrolyte) termed the half-wave potential.
In order to obtain E1/2 from the above equation, we plot a graph of ln[(id-i)/i] against E. The intercept on the x-axis gives then an accurate value of E1/2. The slope of the obtained straight line is equal to nF/RT from which n is determined.
Chitin → Hitin
Chitin is a nitrogen-containing linear polysaccharide of ß(1->4) linked units of N-acetyl-ß-d-glucosamine. The structure of chitin is similar to cellulose except for the replacement hydroxyl group (-OH) at the carbon 2 with an acetyl amine group (–NH–CO–CH3). Chitin is the main component of the exoskeleton, or outer covering of insects, crustaceans, and arachnids. It is also found in the cell walls of certain fungi and algae. After cellulose, chitin is the second most abundant biopolymer in nature. It is insoluble in water, organic solvents, weak acids and lyes.
Cystine → Cistin
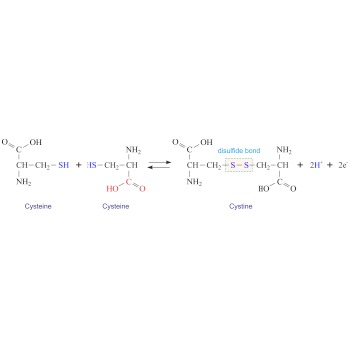
Cystine (C6H12N2O4S2) is a dimer of cysteine. It is formed by the oxidation of the thiol groups (-SH) of two cysteines generating a disulphide bridge (-S-S-). Cystine is a white crystalline solid that is slightly soluble in water. Cystine is particularly abundant in skeletal and connective tissues and in hair, horn, and wool.
Dipeptide → Dipeptid
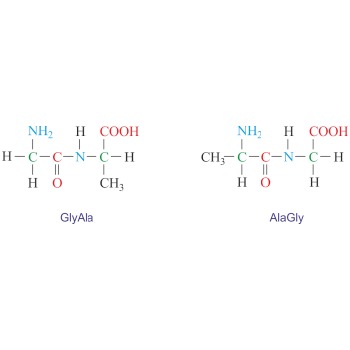
Dipeptide is an organic compound formed when two amino acids are joined by a peptide bond. Depending on which groups of amino acids are involved in the peptide bond four dipeptides can be formed from two different amino acids. For example, glycine (Gly) and alanine (Ala) can give two symmetrical dipeptides (GlyGly and AlaAla) and two unsymmetrical dipeptides (GlyAla and AlaGly). The naming is done by reading the sequence from the N-terminus to the C-terminus.
Citing this page:
Generalic, Eni. "Skupine periodnog sustava." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table