isothermal process → izotermni proces
Isothermal process is a thermodynamic process in which the temperature of the system does not change.
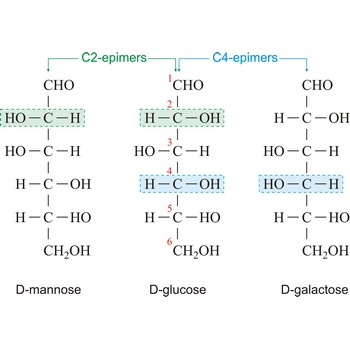
epimer → epimer
Epimers are diastereoisomers that have the opposite configuration at only one of two or more chiral centers present in the respective molecular entities. For example D-glucose and D-mannose, which differ only in the stereochemistry at C-2, are epimers, as are D-glucose and D-galactose (which differ at C-4).
face-centered cubic lattice → plošno centrirana kubična rešetka
Face-centered cubic lattice (fcc or cubic-F), like all lattices, has lattice points at the eight corners of the unit cell plus additional points at the centers of each face of the unit cell. It has unit cell vectors a =b =c and interaxial angles α=β=γ=90°.
The simplest crystal structures are those in which there is only a single atom at each lattice point. In the fcc structures the spheres fill 74 % of the volume. The number of atoms in a unit cell is four (8×1/8 + 6×1/2 = 4). There are 26 metals that have the fcc lattice.
law of conservation of energy → zakon o očuvanju energije
Law of conservation of energy: In an isolated system energy can be transferred from one form to another but the total energy of the system remains constant.
Le Chatelier’s principle → Le Chatelierov princip
The idea that a system at equilibrium will respond to a stress placed upon it in such a manner as to partially offset that stress. The principle was first stated in 1888 by the French physical chemist Henri Le Chatelier (1850-1936).
Mannich reaction → Mannichova reakcija
Mannich reaction is a process in which hydrogen atoms in organic compounds are replaced with a methyl group.
mercaptides → merkaptidi
Mercaptides are derivatives of mercaptanes in which hydrogen from the SH-group is replaced with a metal atom.
Navier-Stokes equations → Navier-Stokesove jednadžbe
Navier-Stokes equations are a set of complex equations for the motion of a viscous fluid subject to external forces.
Citing this page:
Generalic, Eni. "Skupine periodnog sustava." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table