polonium → polonij
Polonium was discovered by Marie Curie (Poland) in 1898. Named for Poland, native country of Marie Curie. It is silvery-grey, extremely rare, radioactive metal. Soluble in dilute acids. Highly toxic. Severe radiotoxicity. Carcinogen. Polonium occurs in pitchblende. Produced by bombarding bismuth with neutrons. Used in industrial equipment that eliminates static electricity caused by such processes as rolling paper, wire and sheet metal.
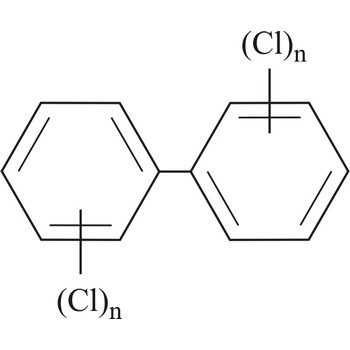
polychlorinated biphenyls → poliklorirani bifenili
Polychlorinated biphenyls (PCB) are poisonous and carcinogenic compounds. They are pollutants of both live and inanimate environment, and they are used as insulation and refrigerating materials in transformers and condensers.
polysaccharide → polisaharid
Polysaccharides are compounds consisting of a large number of simple sugars (monosaccharides) linked together by glycosidic bonds. When polysaccharides are composed of a single monosaccharide building block, they are termed homopolysaccharides. Heteropolysaccharides contain two or more different types of monosaccharide. Polysaccharides may have molecular weights of up to several million and are often highly branched. Since they have only the one free anomeric -OH group at the end of a very long chain, polysaccharides aren’t reducing sugars and don’t show noticeable mutarotation. The most common polysaccharides are cellulose, starch, and glycogen.
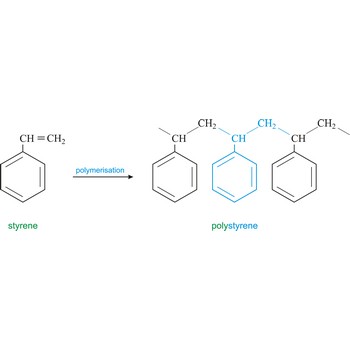
polystyrene → polistiren
Polystyrene is a vinyl polymer. Structurally, it is a long hydrocarbon chain, with a phenyl group attached to every other carbon atom. Polystyrene is produced by free radical vinyl polymerization, from the monomer styrene. Polystyrene or Styrofoam is used in the construction industry as insulating material and for production of containers.
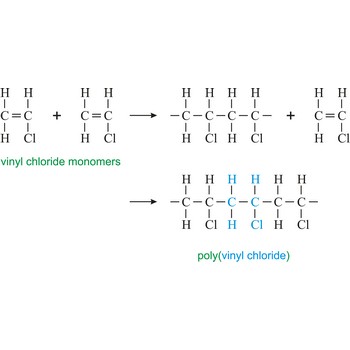
polyvinyl chloride → polivinil klorid
Poly(vinyl chloride) or the PVC is hard and resistant homopolymer produced by the polymerization of the gas vinyl chloride [CH2CHCl]. The pure polymer is hard, brittle and difficult to process, but it becomes flexible when plasticizers are added. After mixing with plasticizers, stabilizers, and pigments, the resin may be fabricated by techniques such as calendering, molding, or extrusion into flexible articles such as raincoats, shower curtains, and packaging films. The resin is not plasticized for use in making rigid products such as water pipe, plumbing fittings, and phonograph records.
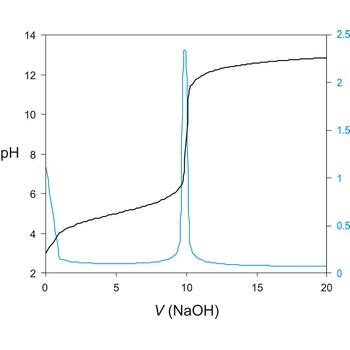
potentiometric titration → potenciometrijska titracija
Potentiometric titration is a volumetric method in which the potential between two electrodes is measured (referent and indicator electrode) as a function of the added reagent volume. Types of potentiometric titrations for the determination of analytes in photoprocessing solutions include acid-base, redox, precipitation, and complexometric.
Potentiometric titrations are preferred to manual titrations, since they are more accurate and precise. They are also more easily adapted to automation, where automated titration systems can process larger volumes of samples with minimal analyst involvement.
A titration curve has a characteristic sigmoid curve. The part of the curve that has the maximum change marks the equivalence point of the titration. The first derivative, ΔE/ΔV, is the slope of the curve, and the endpoint occurs at the volume, V', where ΔE/ΔV has the maximum value.
praseodymium → praseodimij
Praseodymium was discovered by Carl F. Auer von Welsbach (Austria) in 1885. The origin of the name comes from the Greek words prasios didymos meaning green twin. It is silvery white, moderately soft, malleable, ductile metal. Reacts slowly with oxygen. Reacts rapidly with water. Metal ignites and burns readily. Praseodymium is obtained from same salts as neodymium. Used with neodymium to make lenses for glass maker’s goggles since it filters out the yellow light present in glass blowing. Alloyed with magnesium creates a high-strength metal used in aircraft engines. Misch metal, used in the manufacture of pyrophoric alloys for cigarette lighters, contains about 5 % praseodymium metal. (Typically composition of misch metal are Ce:Nd:Pr:La:Other rare earth=50:18:6:22:4).
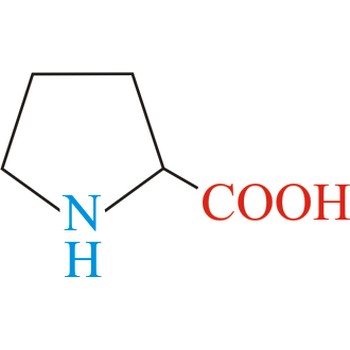
proline → prolin
Proline has an aliphatic side chain with a distinctive cyclic structure. It is unusual because it is conformationally restricted. The secondary amino (imino) group of proline residues is held in a rigid conformation that reduces the structural flexibility of polypeptide regions containing proline. It is not an essential amino acid, which means that the human body can synthesize it.
- Abbreviations: Pro, P
- IUPAC name: pyrrolidine-2-carboxylic acid
- Molecular formula: C5H9NO2
- Molecular weight: 115.13 g/mol
Citing this page:
Generalic, Eni. "Sửa báo cáo khoản vay nước ngoài." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table