supercritical fluid → superkritični fluid
Supercritical fluid is any substance above its critical temperature and critical pressure (see phase diagram). It shows unique properties that are different from those of either gases or liquids under standard conditions. A supercritical fluid has both the gaseous property of being able to penetrate anything, and the liquid property of being able to dissolve materials into their components. Solublity increases with increasing density (i.e. with increasing pressure). An example of this is naphthalene which is practically insoluble in low pressure carbon dioxide. At 100 bar the solubility is 10 g/L and at 200 bar it is 50 g/L. Rapid expansion of supercritical solutions leads to precipitation of a finely divided solid.
supercritical fluid extraction → superkritična fluidna ekstrakcija
Supercritical fluid extractions (SFE) have solvating powers similar to liquid organic solvents, but with higher diffusivities, lower viscosity, and lower surface tension. The main advantages of using supercritical fluids for extractions is that they are inexpensive, contaminant free, and less costly to dispose safely than organic solvents. For non-destructive isolation choose SFE, which is simply the best technology for sensitive raw materials. For these reasons supercritical carbon dioxide (scCO2) is the reagent used to extract caffeine from coffee and tea. Its gaslike behavior allows it to penetrate deep into the green coffee beans, and it dissolves from 97 % to 99 % of the caffeine present.
superfluid helium → superfluidni helij
Superfluidity in helium-4 was discovered in 1938 by the Soviet physicist Pyotr Leonidovich Kapitsa. Helium-4 exhibits superfluidity when it is cooled below 2.18 K (-270.97 C), which is called the lambda (λ) point. At these temperatures, helium-4 exhibits the characteristics of two distinct fluids, one of which appears to flow without friction. An extensive series of experiments showed that in this state of helium, called helium II (He II), there is an apparent enormous rise in heat conductivity, at an increase rate of about three million. Another unusual property of He II is its mobile, rapid flow through capillaries or over the rim of its containment vessel as a thin film that exhibits no measurable viscosity and appears unaffected by the forces of gravity or evaporation and condensation.
accumulator → akumulator
Accumulator (secondary cell, storage battery) is a type of voltaic cell or battery that can be recharged by passing current through it from an external D.C. supply. The charging current reverses the chemical reactions in the cell. The common types are the lead-acid accumulator and the nickel-cadmium cell.
acid → kiselina
Acid is a type of compound that contains hydrogen and dissociates in water to produce positive hydrogen ions. The reaction for an acid HA is commonly written:
In fact, the hydrogen ion (the proton) is solvated, and the complete reaction is:
This definition of acids comes from the Arrhenius theory. Such acids tend to be corrosive substances with a sharp taste, which turn litmus red and produce colour changes with other indicators. They are referred to as protonic acids and are classified into strong acids, which are almost completely dissociated in water, (e.g. sulphuric acid and hydrochloric acid), and weak acids, which are only partially dissociated (e.g. acetic acid and hydrogen sulphide). The strength of an acid depends on the extent to which it dissociates, and is measured by its dissociation constant.
In the Lowry-Brønsted theory of acids and bases (1923), the definition was extended to one in which an acid is a proton donor (a Brønsted acid), and a base is a proton acceptor (a Brønsted base). An important feature of the Lowry-Brønsted concept is that when an acid gives up a proton, a conjugate base is formed that is capable of accepting a proton.
Similarly, every base produces its conjugate acid as a result of accepting a proton.
For example, acetate ion is the conjugate base of acetic acid, and ammonium ion is the conjugate acid of ammonia.
As the acid of a conjugate acid/base pair becomes weaker, its conjugate base becomes stronger and vice versa.
A further extension of the idea of acids and bases was made in the Lewis theory. In this, a G. N. Lewis acid is a compound or atom that can accept a pair of electrons and a Lewis base is one that can donate an electron pair. This definition encompasses "traditional" acid-base reactions, but it also includes reactions that do not involve ions, e.g.
in which NH3 is the base (donor) and BCl3 the acid (acceptor).
AMU → AMU
AMU or atomic mass unit is a unit of mass used to express relative atomic masses. It is equal to 1/12 of the mass of an atom of the isotope carbon-12 and is equal to 1.66 033×10-27 kg. This unit superseded both the physical and a chemical mass unit based on oxygen-16 and is sometimes called the unified mass unit or the dalton.
aluminium → aluminij
Aluminium was discovered by Friedrich Wöhler (Germany) in 1827. The origin of the name comes from the Latin word alumen meaning alum. It is soft, lightweight, silvery-white metal. Exposed surfaces quickly form protective oxide coating. Metal reacts violently with oxidants. Third most abundant element in the earth’s crust. Aluminium is the most abundant metal to be found in the earth’s crust, but is never found free in nature. Aluminium is obtained by electrolysis from bauxite. Used for many purposes from airplanes to beverage cans. Too soft in its pure form so less than 1 % of silicon or iron is added, which hardens and strengthens it.
anomer → anomer
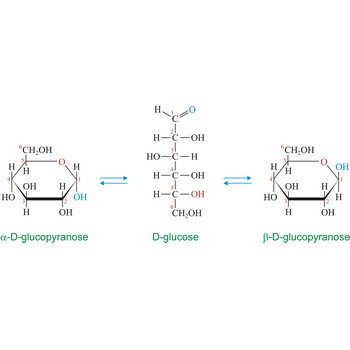
Anomers are diastereoisomers of cyclic forms of sugars or similar molecules differing in the configuration at the anomeric carbon (C-1 atom of an aldose or the C-2 atom of a 2-ketose). The cyclic forms of carbohydrates can exist in two forms, α- and β- based on the position of the substituent at the anomeric center. Anomer are designated α if the configuration at the anomeric carbon is the same as that at the reference asymmetric carbon in a Fischer projection. If the configuration differs the anomer is designated β. For example, α-D-glucopyranose and β-D-glucopyranose, the two cyclic forms of glucose, are anomers.
arginine → arginin
Arginine is an electrically charged amino acids with basic side chains. It is one of the least frequent amino acids. As a group the charged amino acids are important for making proteins soluble. These residues are generally located on the surface of the protein. Arginine is well designed to bind the phosphate anion, and is often found in the active centers of proteins that bind phosphorylated substrates. As a cation, arginine, as well as lysine, plays a role in maintaining the overall charge balance of a protein. Although arginine is considered an essential amino acid (it must be obtained through the diet), this is true only during the juvenile period in humans.
- Abbreviations: Arg, R
- IUPAC name: 2-amino-5-(diaminomethylideneamino)pentanoic acid
- Molecular formula: C6H14N4O2
- Molecular weight: 174.20 g/mol
blast furnace → visoka peć
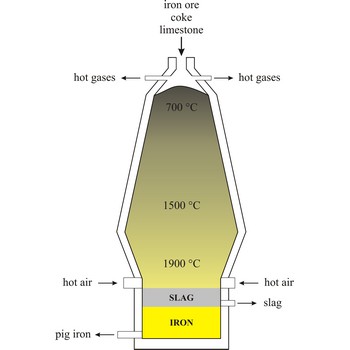
Blast furnace is a furnace for smelting of iron from iron oxide ores (hematite, Fe2O3 or magnetite, Fe3O4). Coke, limestone and iron ore are poured in the top, which would normally burn only on the surface. The hot air blast to the furnace burns the coke and maintains the very high temperatures that are needed to reduce the ore to iron. The reaction between air and the fuel generates carbon monoxide. This gas reduces the iron(III) oxide in the ore to iron.
Because the furnace temperature is in the region of 1500 °C, the metal is produced in a molten state and this runs down to the base of the furnace.
The production of iron in a blast furnace is a continuous process. The furnace is heated constantly and is re-charged with raw materials from the top while it is being tapped from the bottom. Iron making in the furnace usually continues for about ten years before the furnace linings have to be renewed.
Citing this page:
Generalic, Eni. "Sửa báo cáo khoản vay nước ngoài." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table