cell potential → potencijal članka
Cell potential (E) is difference between anode and cathode potential. If the cell potential is positive, then the reaction is spontaneous.
equivalent → ekvivalent
Equivalent (eq) is a unit for describing the amount of a chemical species. In contrast to the mole, the amount of a substance contained in one equivalent can vary from reaction to reaction.
chemical change → kemijska promjena
Chemical change is a process which results in the production of one or more new materials. The system within which the process takes place is called a chemical system. A chemical change is also known as a chemical reaction, where one substance is converted into one or more different substances. When sodium and chlorine react to produce sodium chloride, a chemical reaction has taken place.
equivalent weight → ekvivalentna masa
Equivalent weight of a substance participating in a neutralization reaction is that mass of substance (molecule, ion, or paired ion) that either reacts with or supplies 1 mol of hydrogen ions in that reaction.
Equivalent weight of a substance participating in an oxidation/reduction reaction is that weight which directly or indirectly produces or consumes 1 mol of electrons.
explosion → eksplozija
Explosion is a rapid violent chemical reaction that produces large amounts of gas and heat, accompanied by light, sound and a high-pressure shock wave.
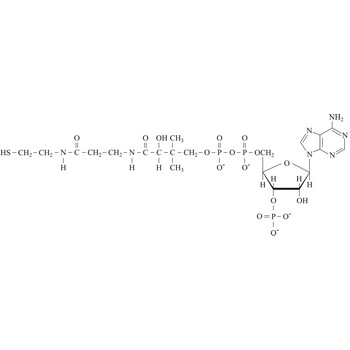
coenzyme a → koenzim a
Coenzyme A (CoA) is an essential metabolic cofactor synthesized from cysteine, pantothenate (vitamin B5), and ATP. CoA plays important roles in many metabolic pathways, including the tricarboxylic acid (TCA) cycle, and the synthesis and oxidation of fatty acids. One of the main functions of CoA is the carrying and transfer of acyl groups. Acylated derivatives (acetyl-CoA) are critical intermediates in many metabolic reactions.
coenzyme q → koenzim q
Coenzyme Q (CoQ) or ubiquinone is any of a group of related quinone-derived compounds that serve as electron carriers in the electron transport chain reactions of cellular respiration. There are some differences in the length of the isoprene unit (in bracket on left) side chain in various species. All the natural forms of CoQ are insoluble in water, but soluble in membrane lipids.
collision theory → teorija sudara
Collision theory is theory that explains how chemical reactions take place and why rates of reaction alter. For a reaction to occur the reactant particles must collide. Only a certain fraction of the total collisions cause chemical change; these are called successful collisions. The successful collisions have sufficient energy (activation energy) at the moment of impact to break the existing bonds and form new bonds, resulting in the products of the reaction. Increasing the concentration of the reactants and raising the temperature bring about more collisions and therefore more successful collisions, increasing the rate of reaction.
fermentation → fermentacija
Fermentation is a class of biochemical reactions that break down complex organic molecules (such as carbohydrates) into simpler materials (such as ethanol, carbon dioxide, and water). Fermentation reactions are catalyzed by enzymes.
Citing this page:
Generalic, Eni. "Reverse reaction." Croatian-English Chemistry Dictionary & Glossary. 29 June 2022. KTF-Split. {Date of access}. <https://glossary.periodni.com>.
Glossary
Periodic Table